

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50077325 2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one::2-(3,4-dihydroxyphenyl)-7-hydroxy-4H-chromen-4-one::3',4',7-trihydroxyflavon::7,3',4'-Trihydroxyflavone::CHEMBL301624
SMILES: Oc1ccc2c(c1)oc(cc2=O)-c1ccc(O)c(O)c1
InChI Key: InChIKey=PVFGJHYLIHMCQD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50077325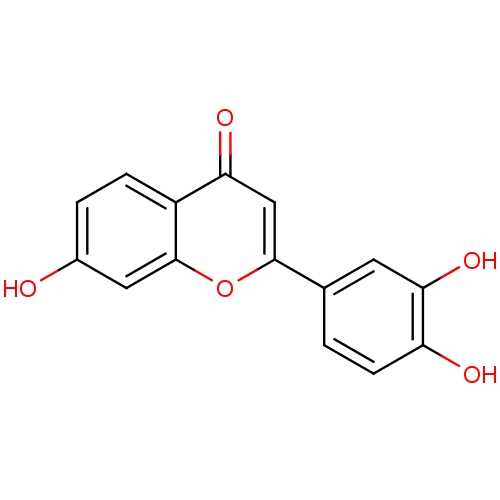 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BU-Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human telomerase from HEK293 cell extracts by Flash-Plate assay | J Med Chem 47: 6466-75 (2004) Article DOI: 10.1021/jm040810b BindingDB Entry DOI: 10.7270/Q2P55N1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (ALR2) (Bos taurus (Cattle)) | BDBM50077325 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Modena Curated by ChEMBL | Assay Description Inhibition of ALR2 (aldose reductase) of bovine lens | J Med Chem 42: 1881-93 (1999) Article DOI: 10.1021/jm980441h BindingDB Entry DOI: 10.7270/Q25X29M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50077325 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu Curated by ChEMBL | Assay Description Inhibition of human 6XHis-tagged TNKS1 SAM-ART domain (1030 to 1317 amino acid residues) by fluorescence assay | J Med Chem 56: 3507-17 (2013) Article DOI: 10.1021/jm3018783 BindingDB Entry DOI: 10.7270/Q24M95W6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50077325 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu Curated by ChEMBL | Assay Description Inhibition of human recombinant ARTD1 by fluorescence assay | J Med Chem 56: 3507-17 (2013) Article DOI: 10.1021/jm3018783 BindingDB Entry DOI: 10.7270/Q24M95W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50077325 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu Curated by ChEMBL | Assay Description Inhibition of human 6XHis-tagged TNKS2 ART domain (946 to 1161 amino acid residues) expressed in Escherichia coli Rosetta2 (DE3) by fluorescence assa... | J Med Chem 56: 3507-17 (2013) Article DOI: 10.1021/jm3018783 BindingDB Entry DOI: 10.7270/Q24M95W6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50077325 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||