

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50078322 CHEMBL3414710
SMILES: [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)C1CC1)c1ccc(F)cc1F
InChI Key: InChIKey=RHJJDGACVHKXNA-JJMVLAAESA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078322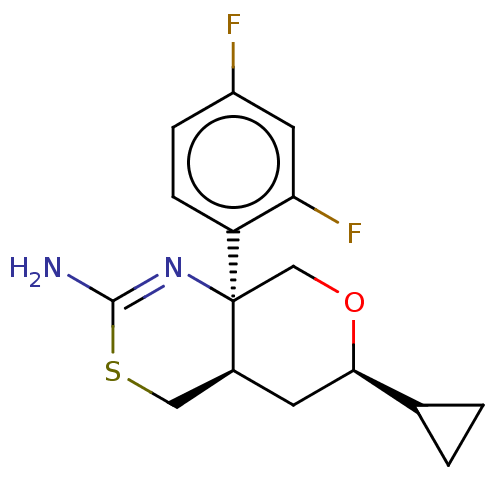 (CHEMBL3414710) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing APP695 assessed as sAPPbeta level after 18 hrs by ELISA | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon Green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50078322 (CHEMBL3414710) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eurofarma Laboratorios S.A. Curated by ChEMBL | Assay Description Inhibition of CatD (unknown origin) by fluorescence polarization assay | J Med Chem 58: 2678-702 (2015) Article DOI: 10.1021/jm501833t BindingDB Entry DOI: 10.7270/Q2W37Z1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||