

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50084875 8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl)-2,7-dimethylpyrazolo[1,5-a][1,3,5]triazin-4-amine::CHEMBL44698::DMP-696::DMP696::[8-(2,4-dichloro-phenyl)-2,7-dimethyl-pyrazolo[1,5-a][1,3,5]triazin-4-yl]-(2-methoxy-1-methoxymethyl-ethyl)-amine
SMILES: COCC(COC)Nc1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl
InChI Key: InChIKey=MDWRPTOUDPFXKK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875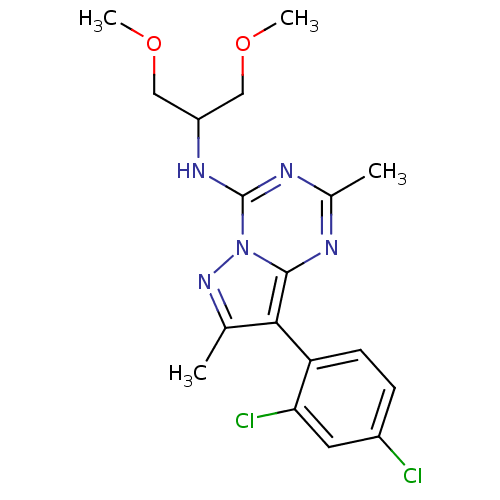 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 | J Med Chem 43: 449-56 (2000) BindingDB Entry DOI: 10.7270/Q2542P9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the binding affinity to human corticotropin releasing factor receptor | J Med Chem 43: 1641-60 (2000) BindingDB Entry DOI: 10.7270/Q29S1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the binding affinity to human corticotropin releasing factor receptor | J Med Chem 43: 1641-60 (2000) BindingDB Entry DOI: 10.7270/Q29S1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CRF-1 receptor | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceuticals Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 57-69 (2003) Article DOI: 10.1124/jpet.102.046128 BindingDB Entry DOI: 10.7270/Q20K273G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from human recombinant CRF1 receptor expressed in CHO cells by SPA | J Med Chem 51: 7273-86 (2009) Article DOI: 10.1021/jm800743q BindingDB Entry DOI: 10.7270/Q2TT4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from human recombinant CRF1 receptor expressed in CHO cells | J Med Chem 51: 7370-9 (2009) Article DOI: 10.1021/jm800744m BindingDB Entry DOI: 10.7270/Q2N87C1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor (Rattus norvegicus (rat)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]ovine-CRF from CRF1 receptor in rat frontal cortex by rapid filtration technique | J Med Chem 52: 4161-72 (2009) Article DOI: 10.1021/jm900302q BindingDB Entry DOI: 10.7270/Q2WW7JM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Curated by ChEMBL | Assay Description Displacement of [125I]CRF from human recombinant CRF1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 5218-21 (2007) Article DOI: 10.1016/j.bmcl.2007.06.077 BindingDB Entry DOI: 10.7270/Q2P55PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor (Rattus norvegicus (rat)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-ovine-CRF from CRF1 receptor in rat frontal cortex homogenate after 2 hrs by gamma counting analysis | Bioorg Med Chem Lett 26: 2184-7 (2016) Article DOI: 10.1016/j.bmcl.2016.03.067 BindingDB Entry DOI: 10.7270/Q2KK9FVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50084875 (8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of corticotropin releasing factor receptor mediated increase in adenylate cyclase activity was evaluated | J Med Chem 43: 1641-60 (2000) BindingDB Entry DOI: 10.7270/Q29S1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||