

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50088507 CHEBI:63804::CHEMBL1908021
SMILES: Oc1ccc(cc1)C1(NC(=O)NC1=O)c1ccccc1
InChI Key: InChIKey=XEEDURHPFVXALT-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uridine-5'-diphosphoglucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088507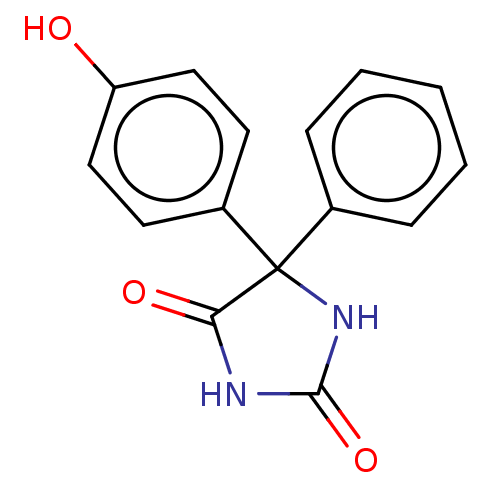 (CHEBI:63804 | CHEMBL1908021) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine-5'-diphosphoglucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088507 (CHEBI:63804 | CHEMBL1908021) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Homo sapiens (Human)) | BDBM50088507 (CHEBI:63804 | CHEMBL1908021) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C delta | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Homo sapiens (Human)) | BDBM50088507 (CHEBI:63804 | CHEMBL1908021) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of protein kinase C epsilon expressed in Sf-9 cells | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Homo sapiens (Human)) | BDBM50088507 (CHEBI:63804 | CHEMBL1908021) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Homo sapiens (Human)) | BDBM50088507 (CHEBI:63804 | CHEMBL1908021) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of protein kinase C epsilon expressed in Sf-9 cells | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||