

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50093173 CHEMBL309830::Derivative of piperazine-1-carboxylic acid 5-(piperazine-1-carbonyloxy)-cyclooctyl ester
SMILES: [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1
InChI Key: InChIKey=UKPPUXUTDBVVBU-RNPORBBMSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase (Homo sapiens (Human)) | BDBM50093173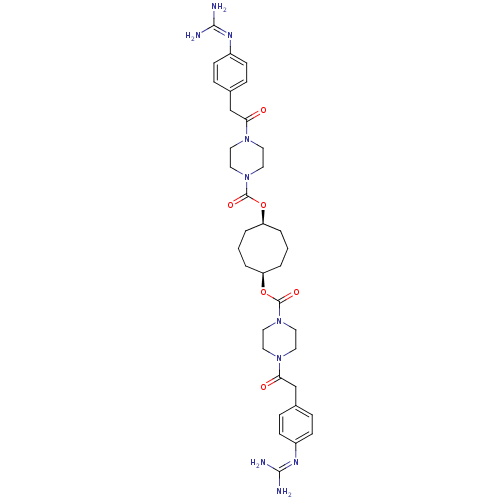 (CHEMBL309830 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM50093173 (CHEMBL309830 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against trypsin | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093173 (CHEMBL309830 | Derivative of piperazine-1-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against thrombin | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093173 (CHEMBL309830 | Derivative of piperazine-1-carboxyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against plasmin | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||