

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50095148 CHEMBL3589535
SMILES: OC(=O)CC(=O)Nc1nc2CC[C@@H](Cc2s1)NC(=O)c1cc(Br)c(Br)[nH]1
InChI Key: InChIKey=QNZTYBLLVQDSEL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA Gyrase (Escherichia coli (strain K12)) | BDBM50095148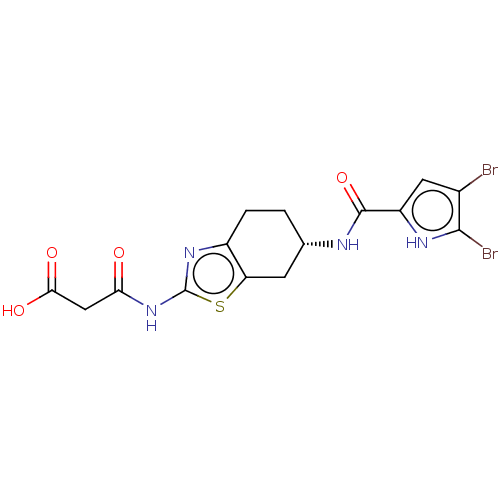 (CHEMBL3589535) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using relaxed pNO1 plasmid and biotinylated oligonucelotide incubated for 30 mins by fluorescence based DNA... | J Med Chem 58: 5501-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00489 BindingDB Entry DOI: 10.7270/Q2MP551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Gyrase (Escherichia coli (strain K12)) | BDBM50095148 (CHEMBL3589535) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Binding affinity to Escherichia coli DNA gyrase subunit B N-terminal 24 kDa domain by SPR assay | J Med Chem 58: 5501-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00489 BindingDB Entry DOI: 10.7270/Q2MP551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Topoisomerase IV (Staphylococcus aureus) | BDBM50095148 (CHEMBL3589535) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 using relaxed pNO1 plasmid and biotinylated oligonucelotide incubated for 30 mins by fluoresc... | J Med Chem 58: 5501-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00489 BindingDB Entry DOI: 10.7270/Q2MP551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Gyrase (Staphylococcus aureus) | BDBM50095148 (CHEMBL3589535) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase using relaxed pNO1 plasmid and biotinylated oligonucelotide incubated for 30 mins by fluorescence base... | J Med Chem 58: 5501-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00489 BindingDB Entry DOI: 10.7270/Q2MP551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||