

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50099352 4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazol-4-yl]-2-p-tolyloxy-pyrimidine::CHEMBL14682
SMILES: Cc1ccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)cc1
InChI Key: InChIKey=MYXXRVIYUKHKPI-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50099352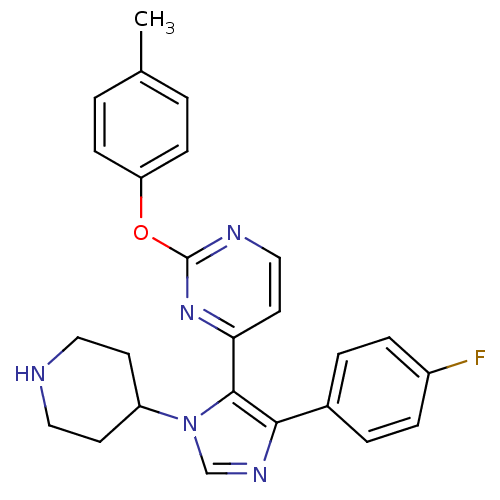 (4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 1123-6 (2001) BindingDB Entry DOI: 10.7270/Q2028QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50099352 (4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of p38alpha | Eur J Med Chem 43: 830-8 (2008) Article DOI: 10.1016/j.ejmech.2007.06.009 BindingDB Entry DOI: 10.7270/Q28052ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and extra-terminal motif (BET) (Homo sapiens (Human)) | BDBM50099352 (4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human BRDT bromodomain 1 (unknown origin) expressed in Escherichia coli Bl21(DE3) by fluorescence anisotropy | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and extra-terminal motif (BET) (Homo sapiens (Human)) | BDBM50099352 (4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human BRDT bromodomain 2 (unknown origin) expressed in Escherichia coli Bl21(DE3) by fluorescence anisotropy | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50099352 (4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BRD4 bromodomain 1 (unknown origin) expressed in Escherichia coli Bl21(DE3) by fluorescence anisotropy | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||