

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50099903 4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulfanyl-thiophene-2-carboxamidine::CHEMBL29205
SMILES: COc1cccc(c1)-c1csc(n1)-c1cc(sc1SC)C(N)=N
InChI Key: InChIKey=HFUGBKCICWUZIJ-UHFFFAOYSA-N
Data: 7 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complement C1s (Homo sapiens (Human)) | BDBM50099903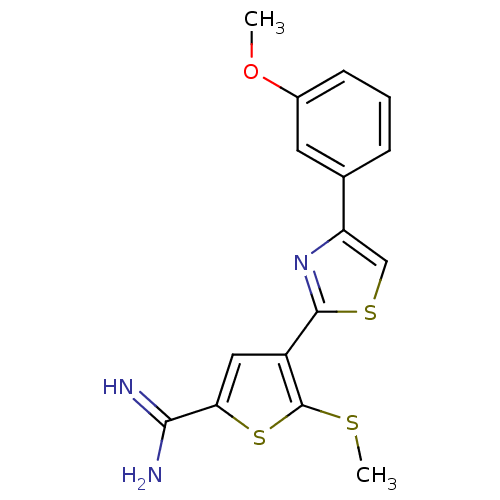 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc Curated by ChEMBL | Assay Description In vitro binding affinity towards human Complement C1s subcomponent | Bioorg Med Chem Lett 14: 3043-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.034 BindingDB Entry DOI: 10.7270/Q2K35T33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM50099903 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Trypsin | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50099903 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against tissue plasminogen activator | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50099903 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against plasmin | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50099903 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Thrombin (no observable inhibition at this screening concentration) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin (Homo sapiens (Human)) | BDBM50099903 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Serine protease chymotrypsin (no observable inhibition at this screening concentration) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099903 (4-[4-(3-Methoxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||