

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50105416 CHEMBL328551::N-((E)-3-Phenyl-allyl)-N-prop-2-ynyl-hydrazine
SMILES: NN(C\C=C\c1ccccc1)CC#C
InChI Key: InChIKey=TWLCPZOKHROBDM-RMKNXTFCSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50105416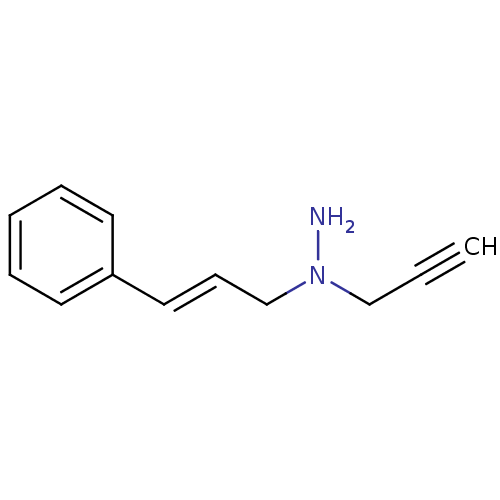 (CHEMBL328551 | N-((E)-3-Phenyl-allyl)-N-prop-2-yny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50105416 (CHEMBL328551 | N-((E)-3-Phenyl-allyl)-N-prop-2-yny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||