

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50107343 (4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-3-hydroxy-tetrahydro-furan-2-ylmethyl ester 5-nitro-furan-2-ylmethyl ester::CHEMBL139442
SMILES: CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCc1ccc(o1)[N+]([O-])=O
InChI Key: InChIKey=QJSUJNHXESNBMF-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50107343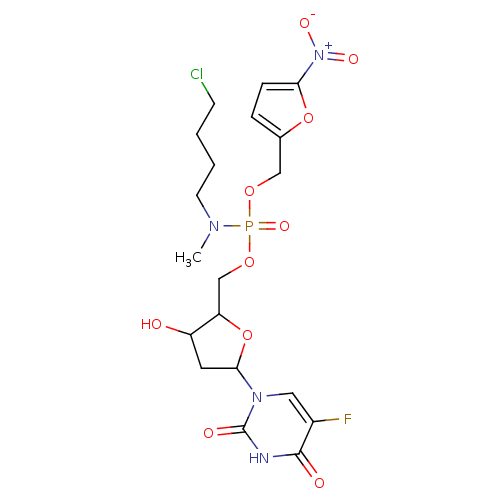 ((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Thymidylate synthase inhibition in L1210 mouse leukemia cells after 2 h treatment | J Med Chem 44: 4475-80 (2001) BindingDB Entry DOI: 10.7270/Q2HM57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50107343 ((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Thymidylate synthase inhibition in thymidine kinase deficient /TK cells after 2 h treatment | J Med Chem 44: 4475-80 (2001) BindingDB Entry DOI: 10.7270/Q2HM57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50107343 ((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Thymidylate synthase inhibition in wild type LM cells after 2 h treatment | J Med Chem 44: 4475-80 (2001) BindingDB Entry DOI: 10.7270/Q2HM57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50107343 ((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Thymidylate synthase inhibition in thymidine kinase deficient LM cells after 2 h treatment | J Med Chem 44: 4475-80 (2001) BindingDB Entry DOI: 10.7270/Q2HM57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||