

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50108642 CHEMBL27725
SMILES: CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cccc(c2C(O)=O)[N+]([O-])=O)cc1
InChI Key: InChIKey=VTPGJVFIRNPJAL-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II receptor (RAT) | BDBM50108642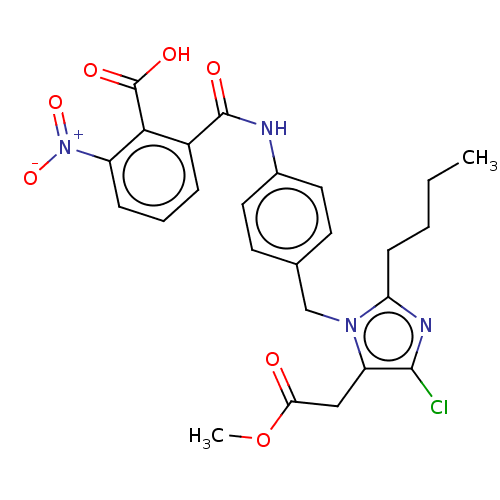 (CHEMBL27725) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||