

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50109484 1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2-yl)-8H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)urea::1-Benzo[1,3]dioxol-5-yl-3-(8-butyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)-urea::CHEMBL352192
SMILES: CCCCn1cc2c(n1)nc(NC(=O)Nc1ccc3OCOc3c1)n1nc(nc21)-c1ccco1
InChI Key: InChIKey=FCRUKTDNWPIBOC-UHFFFAOYSA-N
Data: 9 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50109484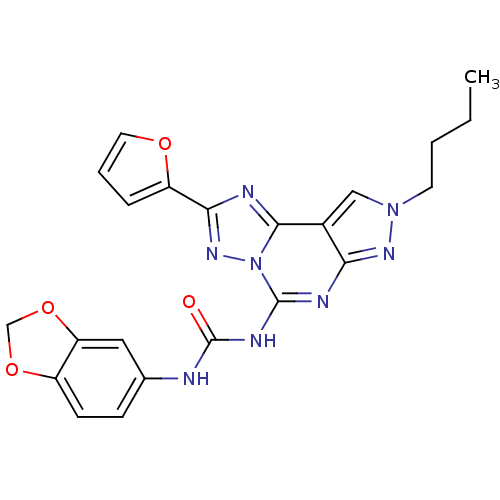 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Padova Curated by ChEMBL | Assay Description Binding affinity for human adenosine A3 receptor | J Med Chem 48: 152-62 (2005) Article DOI: 10.1021/jm049662f BindingDB Entry DOI: 10.7270/Q2183609 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A3 receptor | Bioorg Med Chem Lett 21: 2898-905 (2011) Article DOI: 10.1016/j.bmcl.2011.03.073 BindingDB Entry DOI: 10.7270/Q2VH5P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Padova Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 5259-74 (2009) Checked by Author Article DOI: 10.1016/j.bmc.2009.05.038 BindingDB Entry DOI: 10.7270/Q23N23FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-MRE3008-F20 from human Adenosine A3 receptor expressed in HEK cells; range 0.40-0.62 | J Med Chem 45: 770-80 (2002) BindingDB Entry DOI: 10.7270/Q27W6BHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human adenosine A3 receptor | Citation and Details Article DOI: 10.1007/s00044-013-0849-0 BindingDB Entry DOI: 10.7270/Q22Z18GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human Adenosine A2B receptor expressed in HEK-293 cells; range 57-105 | J Med Chem 45: 770-80 (2002) BindingDB Entry DOI: 10.7270/Q27W6BHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Padova Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cells | Bioorg Med Chem 17: 5259-74 (2009) Checked by Author Article DOI: 10.1016/j.bmc.2009.05.038 BindingDB Entry DOI: 10.7270/Q23N23FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-SCH-58,261 from human Adenosine A2A receptor expressed in HEK-293 cells; range 332-426 | J Med Chem 45: 770-80 (2002) BindingDB Entry DOI: 10.7270/Q27W6BHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50109484 (1-(benzo[d][1,3]dioxol-5-yl)-3-(8-butyl-2-(furan-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human Adenosine A1 receptor expressed in CHO cells; range 314-536 | J Med Chem 45: 770-80 (2002) BindingDB Entry DOI: 10.7270/Q27W6BHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||