

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50116882 CHEMBL3612932
SMILES: [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NCCC)nc(nc12)C#Cc1ccc(Cl)s1)C(=O)NC
InChI Key: InChIKey=CPDWVNNYXONUCC-YWNJHDJRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116882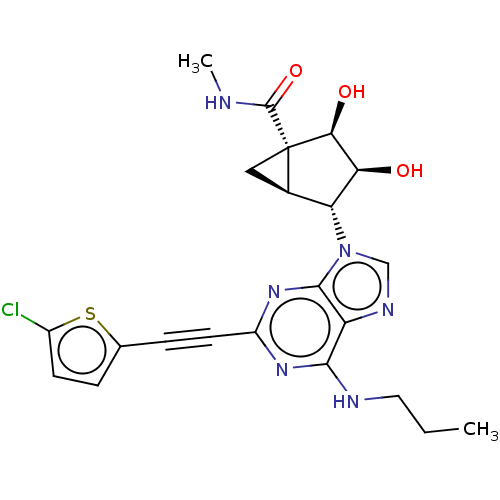 (CHEMBL3612932) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Canis lupus familiaris) | BDBM50116882 (CHEMBL3612932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to canine adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Mus musculus) | BDBM50116882 (CHEMBL3612932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to mouse adenosine A3 receptor expressed in HEK293 cell membranes | ACS Med Chem Lett 6: 804-8 (2015) BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor and beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50116882 (CHEMBL3612932) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibit of purified bovine Farnesyl protein transferase. | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50116882 (CHEMBL3612932) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Enhancing of [3H]WIN35,428 binding to recombinant human DAT expressed in HEK293 cell membranes pre-incubated for 10 mins before radioligand addition ... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50116882 (CHEMBL3612932) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Enhancing of [125I]RTI-55 binding to recombinant human NET expressed in HEK293 cell membranes preincubated for 10 mins followed by radioligand additi... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50116882 (CHEMBL3612932) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Enhancing of [125I]RTI-55 binding to recombinant human DAT expressed in HEK293 cell membranes preincubated for 10 mins followed by radioligand additi... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50116882 (CHEMBL3612932) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vitro binding affinity to displace [3H]spiperone from the cloned human dopamine receptor D2 long in CHO cells | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||