

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50117909 CHEMBL278176::N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-ylmethyl)-2-propyl-phenoxy]-acetyl}-4-isopropyl-benzenesulfonamide
SMILES: CCCc1cc(Cn2c(CC)nc3c(C)cc(C)nc23)ccc1OC(C(=O)NS(=O)(=O)c1ccc(cc1)C(C)C)c1ccc2OCOc2c1
InChI Key: InChIKey=NTQWAQFOJQQOTK-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EDNRB (Homo sapiens (Human)) | BDBM50117909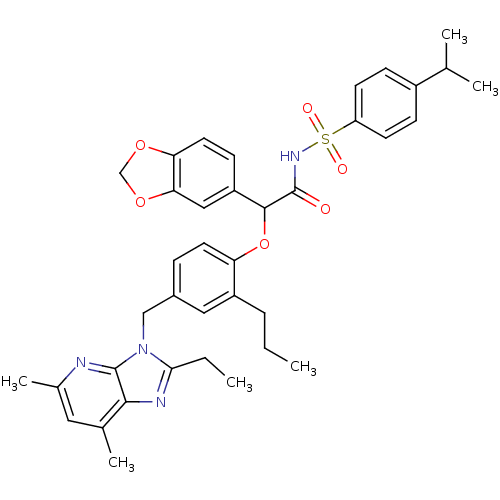 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human Endothelin B receptor (ETB) | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117909 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human Endothelin A receptor (ETA) | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50117909 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50117909 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at type 1 angiotensin 2 receptor | Bioorg Med Chem 20: 4661-7 (2012) Article DOI: 10.1016/j.bmc.2012.06.011 BindingDB Entry DOI: 10.7270/Q2G73FS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EDNRB (Homo sapiens (Human)) | BDBM50117909 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human endothelin B (ETB) receptor expressed in CHO cells | Bioorg Med Chem Lett 5: 1155-1158 (1995) Article DOI: 10.1016/0960-894X(95)00186-W BindingDB Entry DOI: 10.7270/Q2DF6R5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117909 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at endothelin receptor subtype A | Bioorg Med Chem 20: 4661-7 (2012) Article DOI: 10.1016/j.bmc.2012.06.011 BindingDB Entry DOI: 10.7270/Q2G73FS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117909 (CHEMBL278176 | N-{2-Benzo[1,3]dioxol-5-yl-2-[4-(2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human endothelin A (ETA) receptor expressed in CHO cells | Bioorg Med Chem Lett 5: 1155-1158 (1995) Article DOI: 10.1016/0960-894X(95)00186-W BindingDB Entry DOI: 10.7270/Q2DF6R5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||