

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50118235 Brilliant Blue::CHEMBL423337
SMILES: CCOc1ccc(Nc2ccc(cc2)[C+](c2ccc(cc2C)N(CC)Cc2cccc(c2)S([O-])(=O)=O)c2ccc(cc2C)N(CC)Cc2cccc(c2)S([O-])(=O)=O)cc1
InChI Key: InChIKey=DNGMCANIVMBBIY-UHFFFAOYSA-M
Data: 2 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM50118235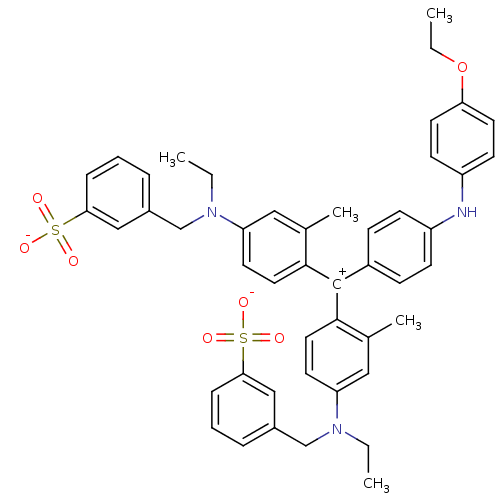 (Brilliant Blue | CHEMBL423337) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 7 (P2X7) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50118235 (Brilliant Blue | CHEMBL423337) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Evaluated for agonist activity against phospholipase C coupled P2Y purinoceptor 1 (P2Y1) of turkey erythrocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||