

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50120439 CHEMBL3617975::US9290459, 47.1::US9670166, 47.1
SMILES: CNC(=O)N1[C@@H](C2=C(CCC2=O)N(C1=O)c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N
InChI Key: InChIKey=RNEGVTLNXUPOAS-OAQYLSRUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50120439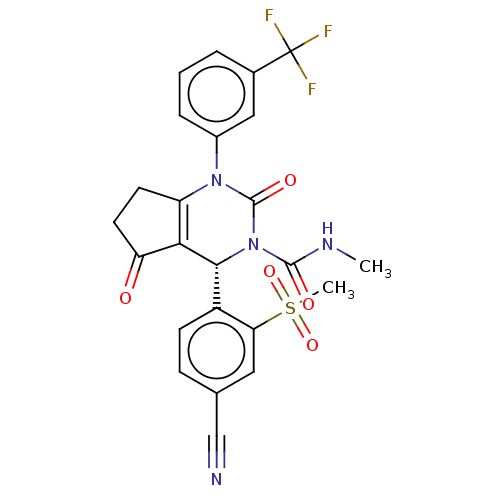 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | <1 | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Various concentrations of the neutrophil elastase inhibitor are incubated with plasma. Subsequently, the enzyme activity is measured using the fluoro... | US Patent US9290459 (2016) BindingDB Entry DOI: 10.7270/Q2KS6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9290459 (2016) BindingDB Entry DOI: 10.7270/Q2KS6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of cytochrome P450 2C9-isoenzyme catalysed hydroxylation of Diclofenac by the test compound is assayed at 37° C. with human liver ... | US Patent US9290459 (2016) BindingDB Entry DOI: 10.7270/Q2KS6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of cytochrome P450 2C19-isoenzyme catalysed hydroxylation of Mephenytoin by the test compound is assayed at 37° C. with human live... | US Patent US9290459 (2016) BindingDB Entry DOI: 10.7270/Q2KS6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of cytochrome P450 2C8-isoenzyme catalysed deethylation of Amodiaquine by the test compound is assayed at 37° C. with human liver ... | US Patent US9290459 (2016) BindingDB Entry DOI: 10.7270/Q2KS6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of neutrophil elastase in human plasma using MeOSuc-Ala-Ala-Pro-Val-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 4370-81 (2015) BindingDB Entry DOI: 10.7270/Q29Z96PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50120439 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeO-Succ-Ala-Ala-Pro-Val-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem Lett 25: 4370-81 (2015) BindingDB Entry DOI: 10.7270/Q29Z96PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||