

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50122764 2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-4-ylmethyl)-3H-imidazol-1-ium; chloride::5-bromo-6-[(2-aminoimidazol-1-yl)methyl]uracil hydrochloride::CHEMBL122679::CHEMBL65986
SMILES: Nc1[nH+]ccn1Cc1[nH]c(=O)[nH]c(=O)c1Br
InChI Key: InChIKey=XUBIFQIUVKXXLR-UHFFFAOYSA-O
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122764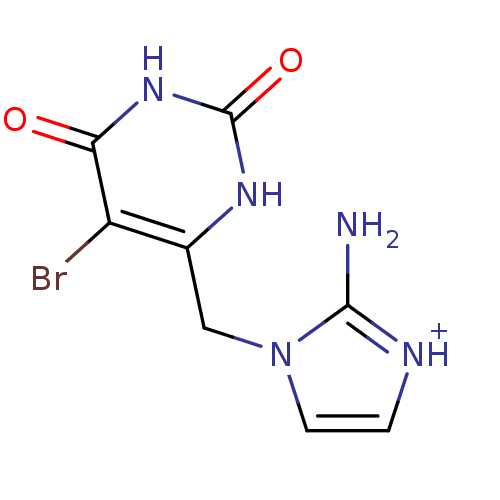 (2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase | J Med Chem 46: 207-9 (2003) Article DOI: 10.1021/jm020964w BindingDB Entry DOI: 10.7270/Q24X574R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122764 (2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory concentration against human thymidine phosphorylase | J Med Chem 48: 392-402 (2005) Article DOI: 10.1021/jm049494r BindingDB Entry DOI: 10.7270/Q2445N8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122764 (2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122764 (2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Eur J Med Chem 43: 1248-60 (2008) Article DOI: 10.1016/j.ejmech.2007.07.015 BindingDB Entry DOI: 10.7270/Q2Z31ZDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||