 Found 8 hits for monomerid = 50126142
Found 8 hits for monomerid = 50126142 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142
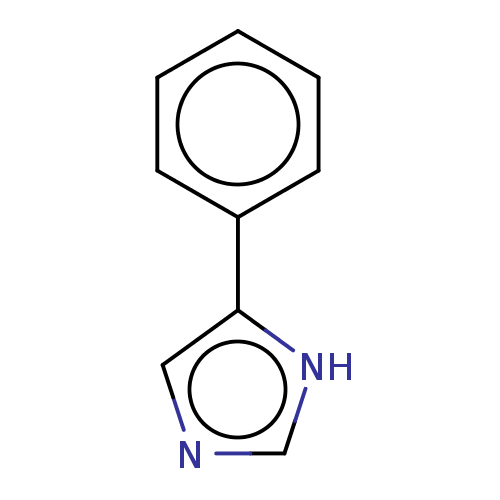
(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado College
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) |
J Med Chem 58: 8762-82 (2015)
BindingDB Entry DOI: 10.7270/Q2C82C3F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy, Fudan University, Shanghai 201203, China.
Curated by ChEMBL
| Assay Description
Inhibition of human IDO expressed in Escherichia coli BL21(DE3) cells assessed as inhibition of kynurenine production using L-tryptophan as substrate... |
Bioorg Med Chem 25: 3780-3791 (2017)
Article DOI: 10.1016/j.bmc.2017.05.017
BindingDB Entry DOI: 10.7270/Q2QJ7KQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation
Curated by ChEMBL
| Assay Description
Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... |
J Med Chem 62: 6705-6733 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00662 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
J Med Chem 62: 8784-8795 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00942 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
J Med Chem 62: 8784-8795 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00942 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of full-length human IDO1 expressed in Escherichia coli Rosetta (DE3) using L-Trp substrate by HPLC analysis |
J Med Chem 62: 8784-8795 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00942 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126142

(4-Phenylimidazole | CHEMBL14145 | US11053207, Comp...)Show InChI InChI=1S/C9H8N2/c1-2-4-8(5-3-1)9-6-10-7-11-9/h1-7H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of full-length human IDO1 expressed in Escherichia coli Rosetta (DE3) using L-Trp substrate by HPLC analysis |
J Med Chem 62: 8784-8795 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00942 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data