

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50126669 (2S)-2-[(5-BENZOFURAN-2-YL-THIOPHEN-2-YLMETHYL)-(2,4-DICHLORO-BENZOYL)-AMINO]-3-PHENYL-PROPIONIC ACID::(S)-2-(N-((5-(benzofuran-2-yl)thiophen-2-yl)methyl)-2,4-dichlorobenzamido)-3-phenylpropanoic acid::(S)-2-[(5-Benzofuran-2-yl-thiophen-2-ylmethyl)-(2,4-dichloro-benzoyl)-amino]-3-phenyl-propionic acid::2-[(5-Benzofuran-2-yl-thiophen-2-ylmethyl)-(2,4-dichloro-benzoyl)-amino]-3-phenyl-propionic acid::CHEMBL278785
SMILES: OC(=O)[C@H](Cc1ccccc1)N(Cc1ccc(s1)-c1cc2ccccc2o1)C(=O)c1ccc(Cl)cc1Cl
InChI Key: InChIKey=YBULOUKTPCHXAL-DEOSSOPVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50126669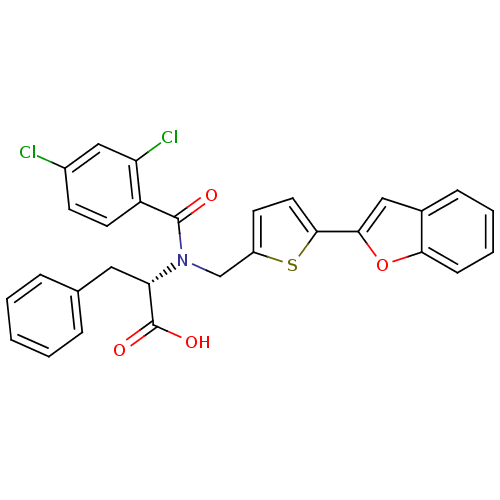 ((2S)-2-[(5-BENZOFURAN-2-YL-THIOPHEN-2-YLMETHYL)-(2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory constant against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM50126669 ((2S)-2-[(5-BENZOFURAN-2-YL-THIOPHEN-2-YLMETHYL)-(2...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S... | J Med Chem 46: 1283-5 (2003) Article DOI: 10.1021/jm0340400 BindingDB Entry DOI: 10.7270/Q2XP74BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||