

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50132477 CHEMBL3632716::US9751873, Example 127
SMILES: C[C@H]1CC[C@H](c2ccccc2)S(=O)(=O)N1Cc1cc(F)c(cc1F)C(CO)CC1COC1
InChI Key: InChIKey=ILKCLQBCDOSHSH-JZZRBVRFSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50132477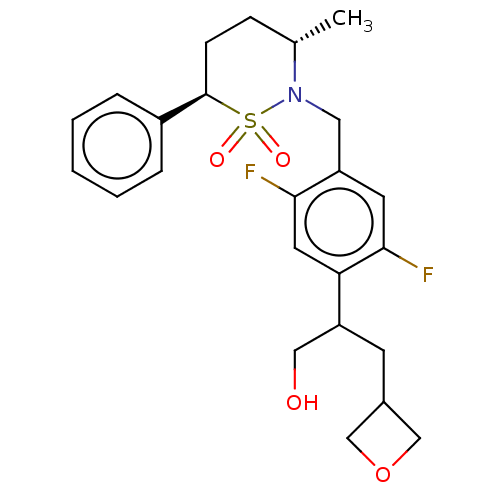 (CHEMBL3632716 | US9751873, Example 127) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptide | ACS Med Chem Lett 6: 958-60 (2015) BindingDB Entry DOI: 10.7270/Q2DN46WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50132477 (CHEMBL3632716 | US9751873, Example 127) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50132477 (CHEMBL3632716 | US9751873, Example 127) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||