

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50134378 (2S)-5-{[(Z)-amino(imino)methyl]amino}-2-(methylamino)pentanoic acid::(2S)-5-{[amino(imino)methyl]amino}-2-(methylamino)pentanoic acid::(S)-5-Guanidino-2-methylamino-pentanoic acid::(S)-5-guanidino-2-(methylamino)pentanoic acid::CHEMBL332148::N-Monomethyl-L-arginine::N~5~-(DIAMINOMETHYLENE)-N~2~-METHYLORNITHINE
SMILES: [#6]-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O
InChI Key: InChIKey=NTWVQPHTOUKMDI-YFKPBYRVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50134378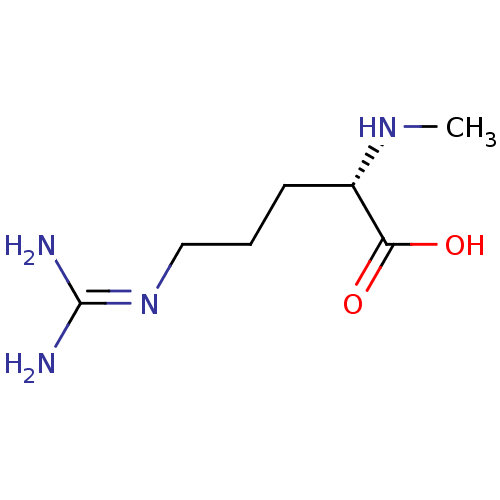 ((2S)-5-{[(Z)-amino(imino)methyl]amino}-2-(methylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against neuronal nitric acid synthase | Bioorg Med Chem Lett 15: 1361-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.013 BindingDB Entry DOI: 10.7270/Q2G160B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50134378 ((2S)-5-{[(Z)-amino(imino)methyl]amino}-2-(methylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against inducible nitric acid synthase | Bioorg Med Chem Lett 15: 1361-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.013 BindingDB Entry DOI: 10.7270/Q2G160B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50134378 ((2S)-5-{[(Z)-amino(imino)methyl]amino}-2-(methylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human eNOS expressed in baculovirus-infected insect sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline preincubat... | J Med Chem 55: 3488-501 (2012) Article DOI: 10.1021/jm300138g BindingDB Entry DOI: 10.7270/Q2KW5H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50134378 ((2S)-5-{[(Z)-amino(imino)methyl]amino}-2-(methylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human nNOS expressed in baculovirus-infected insect sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline preincubat... | J Med Chem 55: 3488-501 (2012) Article DOI: 10.1021/jm300138g BindingDB Entry DOI: 10.7270/Q2KW5H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50134378 ((2S)-5-{[(Z)-amino(imino)methyl]amino}-2-(methylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human iNOS expressed in baculovirus-infected insect sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline preincubat... | J Med Chem 55: 3488-501 (2012) Article DOI: 10.1021/jm300138g BindingDB Entry DOI: 10.7270/Q2KW5H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||