

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50134807 3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]pyrimidin-(4E)-ylideneamine::CHEMBL355905
SMILES: CSc1nc2ccccn2c(=N)c1S(=O)(=O)c1ccccc1
InChI Key: InChIKey=PUHRPYOPXGWCTE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50134807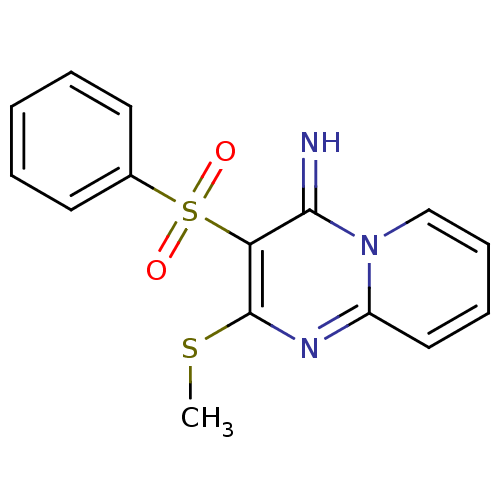 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 6 receptor | J Med Chem 48: 4216-9 (2005) Checked by Author Article DOI: 10.1021/jm050247c BindingDB Entry DOI: 10.7270/Q2736SD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 2C9 | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BzRes | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HeLa cells | Bioorg Med Chem 22: 1782-90 (2014) Article DOI: 10.1016/j.bmc.2014.01.003 BindingDB Entry DOI: 10.7270/Q2PN98M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 2D6 | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 1A2 | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 3A4 with BFC | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute (CDRI) Curated by ChEMBL | Assay Description Antagonist activity at recombinant human 5-HT6 receptor expressed in HEK293 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux... | Bioorg Med Chem 21: 4614-27 (2013) Article DOI: 10.1016/j.bmc.2013.05.040 BindingDB Entry DOI: 10.7270/Q2DJ5JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human Cytochrome P450 19A1 | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50134807 (3-Benzenesulfonyl-2-methylsulfanyl-pyrido[1,2-a]py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 6 receptor using [3H]- LSD as radioligand | J Med Chem 46: 4834-7 (2003) Article DOI: 10.1021/jm034142q BindingDB Entry DOI: 10.7270/Q25T3JWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||