

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50136492 6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline::6-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole::CHEMBL266084
SMILES: COc1ccc2[nH]c3CNCCc3c2c1
InChI Key: InChIKey=QYMDEOQLJUUNOF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50136492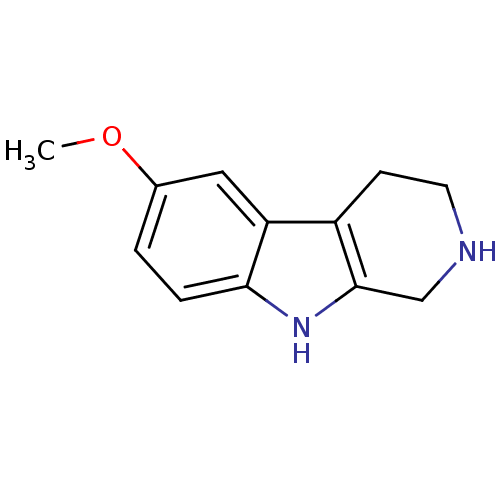 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for rat 5-hydroxytryptamine 2A receptor using [3H]-DOB | Bioorg Med Chem Lett 13: 4421-5 (2003) BindingDB Entry DOI: 10.7270/Q2D21X2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin receptor 2a and 2c (5HT2A and 5HT2C) (Rattus norvegicus (Rat)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards cloned rat 5-hydroxytryptamine 2C receptor from fundus tissue by [3H]-mesulergine displacement. | Bioorg Med Chem Lett 13: 4421-5 (2003) BindingDB Entry DOI: 10.7270/Q2D21X2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards cloned rat 5-hydroxytryptamine 2A receptor by [3H]ketanserin displacement. | Bioorg Med Chem Lett 13: 4421-5 (2003) BindingDB Entry DOI: 10.7270/Q2D21X2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha-2 (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity at alpha-2 adrenergic receptors of rat. | Bioorg Med Chem Lett 14: 999-1002 (2004) Article DOI: 10.1016/j.bmcl.2003.11.078 BindingDB Entry DOI: 10.7270/Q2SB456G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotase (PyrC) (Bacillus anthracis (Firmicute)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.75E+5 | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity to Bacillus anthracis Sterne His-tagged DHOase expressed in Escherichia coli BL21(DE3) Gold cells in presence of N-carbamyl-L-aspart... | Bioorg Med Chem 24: 4536-4543 (2016) Article DOI: 10.1016/j.bmc.2016.07.055 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotase (PyrC) (Bacillus anthracis (Firmicute)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.88E+5 | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity to Bacillus anthracis Sterne His-tagged DHOase expressed in Escherichia coli BL21(DE3) Gold cells by surface plasmon resonance analy... | Bioorg Med Chem 24: 4536-4543 (2016) Article DOI: 10.1016/j.bmc.2016.07.055 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universität Jena Curated by ChEMBL | Assay Description Inhibition of BChE | Bioorg Med Chem Lett 16: 5840-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.067 BindingDB Entry DOI: 10.7270/Q2G73DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universität Jena Curated by ChEMBL | Assay Description Inhibition of AChE | Bioorg Med Chem Lett 16: 5840-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.067 BindingDB Entry DOI: 10.7270/Q2G73DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50136492 (6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | 6...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method | Bioorg Med Chem Lett 22: 2885-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.057 BindingDB Entry DOI: 10.7270/Q2251K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||