

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50139184 4-(2-cyclohexyl-1-ethynyl)-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo[19.2.2.13,7.19,13.015,19]heptacosa-3(27),4,6,9(26),10,12,16,18-octaen-10-yl cyanide::CHEMBL158586
SMILES: O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1C#CC1CCCCC1
InChI Key: InChIKey=OFEALQXVDCUNBG-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geranylgeranyl transferase type I beta subunit/Protein Farnesyltransferase (PFT) (Homo sapiens (Human)) | BDBM50139184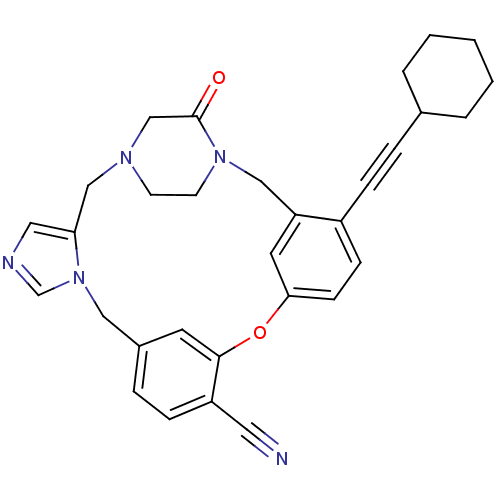 (4-(2-cyclohexyl-1-ethynyl)-23-oxo-8-oxa-1,15,17,21...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Geranylgeranyl transferase type I incorporation of [3H]-GGPP into biotinylated peptide corresponding to the C-terminus of human K... | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Farnesyltransferase (PFT) (Homo sapiens (Human)) | BDBM50139184 (4-(2-cyclohexyl-1-ethynyl)-23-oxo-8-oxa-1,15,17,21...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Farnesyltransferase (PFT) (Homo sapiens (Human)) | BDBM50139184 (4-(2-cyclohexyl-1-ethynyl)-23-oxo-8-oxa-1,15,17,21...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||