

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50140672 (2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid::Beta-lactam compound, 2::CHEMBL25613
SMILES: CC1(C)S[C@@H]2[C@H](CO)C(=O)N2[C@H]1C(O)=O
InChI Key: InChIKey=DJIMYYWAZIOWRD-JCGDXUMPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Acinetobacter baumannii) | BDBM50140672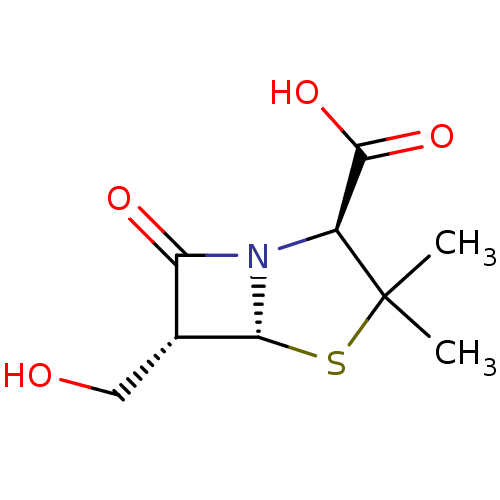 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50140672 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Beta-Lactamase inhibitory activity against representative class A (TEM-1) serine enzyme | Bioorg Med Chem Lett 14: 1299-304 (2004) Article DOI: 10.1016/j.bmcl.2003.12.037 BindingDB Entry DOI: 10.7270/Q22Z14ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase 2 (Bacillus cereus) | BDBM50140672 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of class B (BCII) metallo-beta-lactamase representative enzyme | Bioorg Med Chem Lett 14: 1299-304 (2004) Article DOI: 10.1016/j.bmcl.2003.12.037 BindingDB Entry DOI: 10.7270/Q22Z14ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase L1 (Stenotrophomonas maltophilia) | BDBM50140672 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Beta-Lactamase Inhibition of metallo-beta-lactamase representative class B (L1) enzyme | Bioorg Med Chem Lett 14: 1299-304 (2004) Article DOI: 10.1016/j.bmcl.2003.12.037 BindingDB Entry DOI: 10.7270/Q22Z14ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50140672 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Beta-Lactamase inhibitory activity against representativeclass C (P99) serine enzyme | Bioorg Med Chem Lett 14: 1299-304 (2004) Article DOI: 10.1016/j.bmcl.2003.12.037 BindingDB Entry DOI: 10.7270/Q22Z14ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||