

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50140829 4-(4-Phenethyl-piperazin-1-yl)-6,7,8,9-tetrahydro-pyrimido[4,5-b]indolizine-10-carbonitrile::CHEMBL28098
SMILES: N#Cc1c2CCCCn2c2c(ncnc12)N1CCN(CCc2ccccc2)CC1
InChI Key: InChIKey=WUUUPJQNNUBMKM-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50140829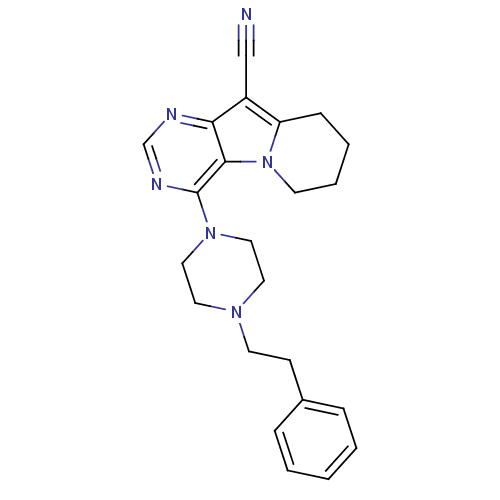 (4-(4-Phenethyl-piperazin-1-yl)-6,7,8,9-tetrahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human transporter MRP1 (Multidrug resistance associated protein 1) expressed in COR.L23/R cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 and 3 (MDR1a/MDR1b) (Mus musculus) | BDBM50140829 (4-(4-Phenethyl-piperazin-1-yl)-6,7,8,9-tetrahydro-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against mouse transporter Pgp (P-glycoprotein) expressed in EMT6/AR1.0 cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family G member 2 (Homo sapiens (Human)) | BDBM50140829 (4-(4-Phenethyl-piperazin-1-yl)-6,7,8,9-tetrahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of BCRP (unknown origin) expressed in MDCK2 cells assessed as pheophorbide A accumulation preincubated for 20 mins measured after 120 mins... | J Med Chem 59: 3018-33 (2016) BindingDB Entry DOI: 10.7270/Q2BG2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50140829 (4-(4-Phenethyl-piperazin-1-yl)-6,7,8,9-tetrahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of MRP1 in human H69AR cells incubated for 20 mins measured every 60 mins by calcein-AM accumulation assay | J Med Chem 59: 3018-33 (2016) BindingDB Entry DOI: 10.7270/Q2BG2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50140829 (4-(4-Phenethyl-piperazin-1-yl)-6,7,8,9-tetrahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of MRP1 in human H69AR cells assessed as daunorubicin accumulation preincubated for 15 mins measured after 180 mins by flow cytometry | J Med Chem 59: 3018-33 (2016) BindingDB Entry DOI: 10.7270/Q2BG2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||