

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142324 ({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-methyl)-phenyl]-2-(1H-tetrazol-5-yl)-propyl]-phenyl}-difluoro-methyl)-phosphonic acid::CHEMBL418703
SMILES: OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1
InChI Key: InChIKey=NFYJBAFWWYMJRQ-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50142324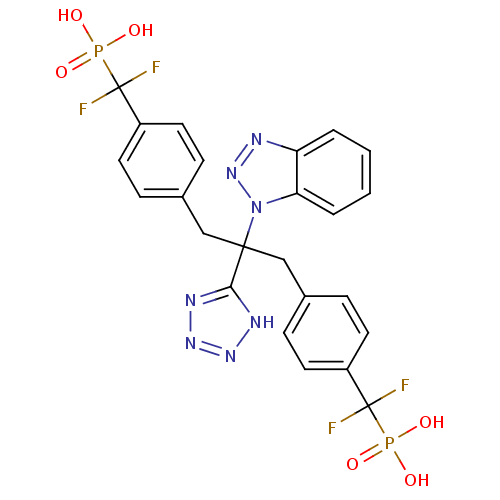 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142324 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50142324 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Leukocyte common antigen (Homo sapiens (Human)) | BDBM50142324 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against CD45 protein-tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||