

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142916 (1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-18-[2-(2-isopropylamino-thiazol-4-yl)-7-methoxy-quinolin-4-yloxy]-2,15-dioxo-3,16-diaza-tricyclo[14.3.0.0*4,6*]nonadec-7-ene-4-carboxylic acid::(1S,4R,6S,14S,18R)-14-cyclopentyloxycarbonylamino-18-[2-(2-isopropylamino-thiazol-4-yl)-7-methoxy-quinolin-4-yloxy]-2,15-dioxo-3,16-diaza-tricyclo[14.3.0.4,6]nonadec-7-ene-4-carboxylic acid::(2R,6S,13aS,14aR,16aS,Z)-6-(cyclopentyloxycarbonylamino)-2-(2-(2-(isopropylamino)thiazol-4-yl)-7-methoxyquinolin-4-yloxy)-5,16-dioxo-1,2,3,5,6,7,8,9,10,11,13a,14,14a,15,16,16a-hexadecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a-carboxylic acid::(Z)-(1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-18-[2-(2-isopropylamino-thiazol-4-yl)-7-methoxy-quinolin-4-yloxy]-2,15-dioxo-3,16-diaza-tricyclo[14.3.0.04,6]nonadec-7-ene-4-carboxylic acid::BILN 2061::CHEMBL297884
SMILES: COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(O)=O)NC(=O)OC3CCCC3)cc(nc2c1)-c1csc(NC(C)C)n1
InChI Key: InChIKey=PJZPDFUUXKKDNB-KNINVFKUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50142916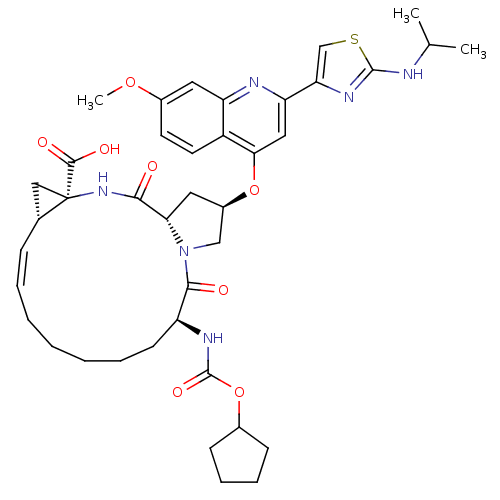 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a NS3 protease | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NS3 protein (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Analysis of the inhibition activation of flNS3 by the inhibitors was performed with the standard assay, allowing the inhibitor to preincubate with NS... | Biochemistry 48: 11592-602 (2009) Article DOI: 10.1021/bi9016928 BindingDB Entry DOI: 10.7270/Q2F47MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein NS3-4A (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV 1a NS3 protease | Bioorg Med Chem 15: 1448-74 (2007) Article DOI: 10.1016/j.bmc.2006.11.003 BindingDB Entry DOI: 10.7270/Q22B8ZVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NS3 Protease (Hepatitis C Virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full-length HCV NS3 protease R155K mutant | J Med Chem 57: 1790-801 (2014) Article DOI: 10.1021/jm301887f BindingDB Entry DOI: 10.7270/Q2V127T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NS3 Protease (Hepatitis C Virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full-length HCV NS3 protease A156T mutant | J Med Chem 57: 1790-801 (2014) Article DOI: 10.1021/jm301887f BindingDB Entry DOI: 10.7270/Q2V127T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease NS3/Non-structural protein 4A (NS3/NS4A) (Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full-length HCV NS3 protease D168V mutant | J Med Chem 57: 1790-801 (2014) Article DOI: 10.1021/jm301887f BindingDB Entry DOI: 10.7270/Q2V127T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein NS3-4A (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||