

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Genome polyprotein | ||
| Ligand | BDBM50142916 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1295506 (CHEMBL3131504) | ||
| Ki | 120±n/a nM | ||
| Citation |  Gising, J; Belfrage, AK; Alogheli, H; Ehrenberg, A; ┼kerblom, E; Svensson, R; Artursson, P; KarlÚn, A; Danielson, UH; Larhed, M; Sandstr÷m, A Achiral pyrazinone-based inhibitors of the hepatitis C virus NS3 protease and drug-resistant variants with elongated substituents directed toward the S2 pocket. J Med Chem57:1790-801 (2014) [PubMed] Article Gising, J; Belfrage, AK; Alogheli, H; Ehrenberg, A; ┼kerblom, E; Svensson, R; Artursson, P; KarlÚn, A; Danielson, UH; Larhed, M; Sandstr÷m, A Achiral pyrazinone-based inhibitors of the hepatitis C virus NS3 protease and drug-resistant variants with elongated substituents directed toward the S2 pocket. J Med Chem57:1790-801 (2014) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Genome polyprotein | |||
| Name: | Genome polyprotein | ||
| Synonyms: | NS3 Protease | ||
| Type: | Protein | ||
| Mol. Mass.: | 18999.15 | ||
| Organism: | Hepatitis C Virus | ||
| Description: | ABC73367 | ||
| Residue: | 181 | ||
| Sequence: |
| ||
| BDBM50142916 | |||
| n/a | |||
| Name | BDBM50142916 | ||
| Synonyms: | (1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-18-[2-(2-isopropylamino-thiazol-4-yl)-7-methoxy-quinolin-4-yloxy]-2,15-dioxo-3,16-diaza-tricyclo[14.3.0.0*4,6*]nonadec-7-ene-4-carboxylic acid | (1S,4R,6S,14S,18R)-14-cyclopentyloxycarbonylamino-18-[2-(2-isopropylamino-thiazol-4-yl)-7-methoxy-quinolin-4-yloxy]-2,15-dioxo-3,16-diaza-tricyclo[14.3.0.4,6]nonadec-7-ene-4-carboxylic acid | (2R,6S,13aS,14aR,16aS,Z)-6-(cyclopentyloxycarbonylamino)-2-(2-(2-(isopropylamino)thiazol-4-yl)-7-methoxyquinolin-4-yloxy)-5,16-dioxo-1,2,3,5,6,7,8,9,10,11,13a,14,14a,15,16,16a-hexadecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a-carboxylic acid | (Z)-(1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-18-[2-(2-isopropylamino-thiazol-4-yl)-7-methoxy-quinolin-4-yloxy]-2,15-dioxo-3,16-diaza-tricyclo[14.3.0.04,6]nonadec-7-ene-4-carboxylic acid | BILN 2061 | CHEMBL297884 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C40H50N6O8S | ||
| Mol. Mass. | 774.925 | ||
| SMILES | COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)[C@H](CCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(O)=O)NC(=O)OC3CCCC3)cc(nc2c1)-c1csc(NC(C)C)n1 |r,c:22| | ||
| Structure | 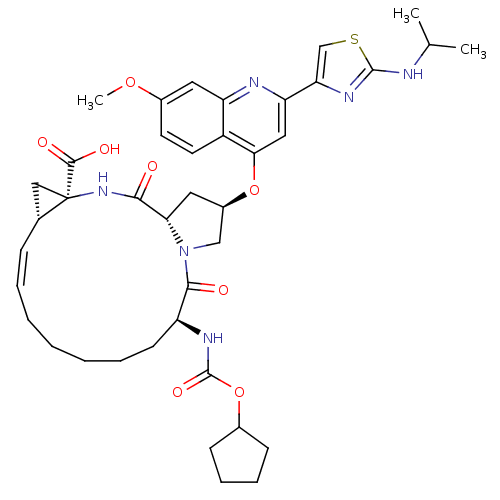
| ||