

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50145829 4-Aminobenzoesaeure::4-aminobenzoic acid::CHEMBL542::PABA::p-Aminobenzoesaeure::p-aminobenzoic acid::para-aminobenzoic acid
SMILES: Nc1ccc(cc1)C(O)=O
InChI Key: InChIKey=ALYNCZNDIQEVRV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50145829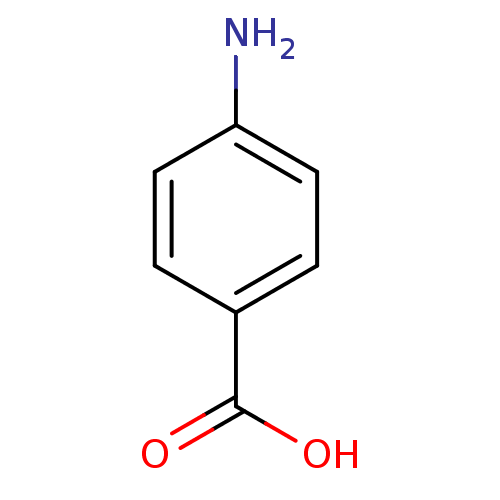 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Dissociation constant for HCV NS3 protease substrate binding site | J Med Chem 47: 2486-98 (2004) Article DOI: 10.1021/jm0305117 BindingDB Entry DOI: 10.7270/Q2FF3RSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Succinate semialdehyde dehydrogenase (Homo sapiens (Human)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity against SSADH | Bioorg Med Chem Lett 16: 592-5 (2005) Article DOI: 10.1016/j.bmcl.2005.10.040 BindingDB Entry DOI: 10.7270/Q29G5MCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-amino-N-butyrate transaminase (Homo sapiens (Human)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity against GABAT | Bioorg Med Chem Lett 16: 592-5 (2005) Article DOI: 10.1016/j.bmcl.2005.10.040 BindingDB Entry DOI: 10.7270/Q29G5MCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Whittier College Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5aplha cells assessed as O-deethylation of ethoxyresorufin in presence of NADP... | Bioorg Med Chem Lett 26: 3243-3247 (2016) BindingDB Entry DOI: 10.7270/Q2XG9T1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiopurine S-methyltransferase (Homo sapiens (Human)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.88E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of purified human kidney thiopurine methyltransferase (TPMT) | J Med Chem 29: 354-8 (1986) BindingDB Entry DOI: 10.7270/Q2445NPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 35.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University Curated by ChEMBL | Assay Description Inhibition of AChE activity in rat brain homogenate using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 20: 521-30 (2011) Article DOI: 10.1016/j.bmc.2011.05.027 BindingDB Entry DOI: 10.7270/Q22B903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydropteroate synthase (Bacillus anthracis) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity to Bacillus anthracis DHPS expressed in Escherichia coli BL21 (DE3) after 30 mins by isothermal titration calorimetry | J Med Chem 53: 166-77 (2010) Article DOI: 10.1021/jm900861d BindingDB Entry DOI: 10.7270/Q2125TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||