

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50147744 1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-yl}-1,3-dihydro-indol-2-one::CHEMBL97979
SMILES: CC(N1CCC(CC1)N1C(=O)Cc2ccccc12)c1ccc(cc1)C1CCCCC1
InChI Key: InChIKey=KVNPCTLYRRUZDP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50147744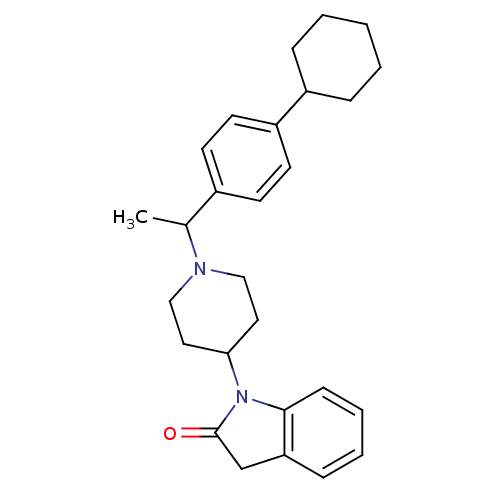 (1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity against human Opioid receptor mu 1 on CHO cell membranes was determined by [3H]DAMGO displacement. | J Med Chem 47: 2973-6 (2004) Article DOI: 10.1021/jm034249d BindingDB Entry DOI: 10.7270/Q2KW5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50147744 (1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity against human Nociceptin receptor on CHO cell membranes by [3H]N/OFQ displacement. | J Med Chem 47: 2973-6 (2004) Article DOI: 10.1021/jm034249d BindingDB Entry DOI: 10.7270/Q2KW5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50147744 (1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity against human Opioid receptor kappa 1 on CHO cell membranes by [3H]U-69593 displacement. | J Med Chem 47: 2973-6 (2004) Article DOI: 10.1021/jm034249d BindingDB Entry DOI: 10.7270/Q2KW5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor protein alpha-4/beta-2 subunit (Rattus norvegicus (Rat)) | BDBM50147744 (1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK292 cells after 3 hrs | J Med Chem 53: 8187-91 (2010) Article DOI: 10.1021/jm1006148 BindingDB Entry DOI: 10.7270/Q2MC9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-3/Beta-4 (Rattus norvegicus (Rat)) | BDBM50147744 (1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR expressed in HEK292 cells after 3 hrs | J Med Chem 53: 8187-91 (2010) Article DOI: 10.1021/jm1006148 BindingDB Entry DOI: 10.7270/Q2MC9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50147744 (1-{1-[1-(4-Cyclohexyl-phenyl)-ethyl]-piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required for stimulation of [35S]-GTP-gammaS, binding to human Nociceptin receptor in cell membranes | J Med Chem 47: 2973-6 (2004) Article DOI: 10.1021/jm034249d BindingDB Entry DOI: 10.7270/Q2KW5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||