

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50148573 (E,E)-1-piperoylpiperidine::1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine::1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-2,4-pentadienoyl]piperidine::1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)penta-2,4-dienoyl]piperidine::1-piperoylpiperidine::CHEMBL43185::N-[(E,E)-piperoyl]piperidine::piperine
SMILES: O=C(\C=C\C=C\c1ccc2OCOc2c1)N1CCCCC1
InChI Key: InChIKey=MXXWOMGUGJBKIW-YPCIICBESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoamine oxidase (Mus musculus) | BDBM50148573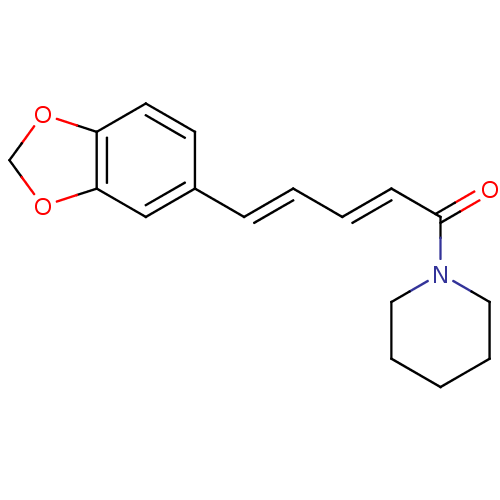 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of mouse brain MAOB | Bioorg Med Chem Lett 20: 537-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.106 BindingDB Entry DOI: 10.7270/Q2000264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Mus musculus) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of mouse brain MAOA | Bioorg Med Chem Lett 20: 537-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.106 BindingDB Entry DOI: 10.7270/Q2000264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of MAOA in rat brain mitochondria | Bioorg Med Chem Lett 20: 537-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.106 BindingDB Entry DOI: 10.7270/Q2000264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of MAOB in rat brain mitochondria | Bioorg Med Chem Lett 20: 537-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.106 BindingDB Entry DOI: 10.7270/Q2000264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
Universidad de Antioquia Curated by ChEMBL | Assay Description Agonist activity at human TRPV1 expressed in tetracycline-stimulated HEK293 cells assessed as increase in intracellular calcium levels by fluorimetri... | Bioorg Med Chem 18: 3299-306 (2010) Article DOI: 10.1016/j.bmc.2010.03.013 BindingDB Entry DOI: 10.7270/Q24M95RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin transepithelial transport (basal to apical) (Digoxin: 5 uM) in Caco-2 cells | J Pharmacol Exp Ther 302: 645-50 (2002) Article DOI: 10.1124/jpet.102.034728 BindingDB Entry DOI: 10.7270/Q2DB82Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Cyclosporin A transepithelial transport (basal to apical) (Cyclosporin A: 1 uM) in Caco-2 cells | J Pharmacol Exp Ther 302: 645-50 (2002) Article DOI: 10.1124/jpet.102.034728 BindingDB Entry DOI: 10.7270/Q2DB82Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
General Hospital of PLA Curated by ChEMBL | Assay Description Inhibition of MAO-A in Sprague-Dawley rat brain homogenate using kynuramine as substrate preincubated for 10 mins measured by fluorimetric assay | Bioorg Med Chem Lett 22: 3343-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.090 BindingDB Entry DOI: 10.7270/Q2JW8FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
General Hospital of PLA Curated by ChEMBL | Assay Description Inhibition of MAO-B in Sprague-Dawley rat brain homogenate using kynuramine as substrate preincubated for 10 mins measured by fluorimetric assay | Bioorg Med Chem Lett 22: 3343-8 (2012) Article DOI: 10.1016/j.bmcl.2012.02.090 BindingDB Entry DOI: 10.7270/Q2JW8FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.24E+4 | n/a | n/a | n/a | n/a |
University of Vienna Curated by ChEMBL | Assay Description Modulation of GABAA alpha1beta2gamma2S receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as potentiation of GABA-induced chlorid... | J Med Chem 57: 5602-19 (2014) Article DOI: 10.1021/jm5002277 BindingDB Entry DOI: 10.7270/Q25X2CXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human microsomal MAO-B expressed in baculovirus infected BTI-TN-5B1-4 cells assessed as reduction in 4-hydroxyquinoline formation using... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of cathepsin D (unknown origin) using hemoglobin as substrate after 30 min by spectrophotometric analysis | Citation and Details Article DOI: 10.1007/s00044-012-0397-z BindingDB Entry DOI: 10.7270/Q29026PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Ohio Medical University Curated by ChEMBL | Assay Description Inhibition of MAO-A assessed as inhibition of kyneuramine conversion to 4-hydroxyquinoline after 20 mins by fluorescence assay | Bioorg Med Chem Lett 22: 7183-8 (2012) Article DOI: 10.1016/j.bmcl.2012.09.056 BindingDB Entry DOI: 10.7270/Q29W0GNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Ohio Medical University Curated by ChEMBL | Assay Description Inhibition of MAO-B assessed as inhibition of kyneuramine conversion to 4-hydroxyquinoline after 20 mins by fluorescence assay | Bioorg Med Chem Lett 22: 7183-8 (2012) Article DOI: 10.1016/j.bmcl.2012.09.056 BindingDB Entry DOI: 10.7270/Q29W0GNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Advanced Sciences Curated by ChEMBL | Assay Description Inhibition of human PMNL 5-LOX using arachidonic acid as substrate after 5 mins by HPLC method | Bioorg Med Chem 27: 3745-3759 (2019) Article DOI: 10.1016/j.bmc.2019.06.040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GITAM University | Assay Description An SX.18MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic/inhibition of various CA isozymes as repo... | J Enzyme Inhib Med Chem 27: 97-100 (2012) Article DOI: 10.3109/14756366.2011.578393 BindingDB Entry DOI: 10.7270/Q2HH6HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GITAM University | Assay Description An SX.18MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic/inhibition of various CA isozymes as repo... | J Enzyme Inhib Med Chem 27: 97-100 (2012) Article DOI: 10.3109/14756366.2011.578393 BindingDB Entry DOI: 10.7270/Q2HH6HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell (A) | Syringe (B) | Cell Links | Syringe Links | Cell + Syr Links | ΔG° kcal/mole | -TΔS° kcal/mole | ΔH° kcal/mole | log K | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|
| Lipoxygenase type I-B (LOX) (Glycine max (Soybean)) | BDBM50148573 ((E,E)-1-piperoylpiperidine | 1-[(2E,4E)-5-(1,3-ben...) | GoogleScholar | CHEBI KEGG PC cid PC sid PDB | -7.48 | 608 | -616 | 5.48 | 8 | 25 | |
Kannur University, Thalassery Campus | Chem Biol Drug Des 85: 715-21 (2015) | |||||||||