

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC1=NC2(CCCCC2)NC(Nc2cccc(Br)c2)=N1
InChI Key: InChIKey=DDIYHVZHGLOJAJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50148831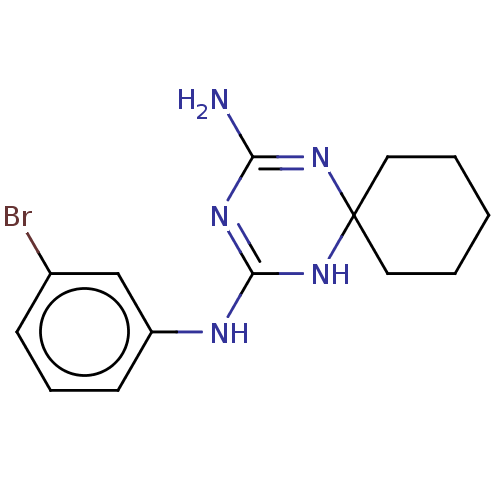 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT2B (unknown origin) after 1.5 hrs by microbeta scintillation counting method | ACS Med Chem Lett 9: 1019-1024 (2018) Article DOI: 10.1021/acsmedchemlett.8b00300 BindingDB Entry DOI: 10.7270/Q2DZ0C0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT2B receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT2C receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from 5-HT2A receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT5A receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT7A receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]GR127543 from 5-HT1D receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT6 receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]GR127543 from 5-HT1B receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from 5-HT3 receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from 5-HT1E receptor (unknown origin) after 1.5 hrs by microbeta scintillation counting method | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHO-K1 cells assessed as calcium flux after 60 mins by FLIPR assay | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50148831 (CHEMBL3770342) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Inhibition of human ERG channel by patch clamp assay | J Med Chem 59: 707-20 (2016) BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50148831 (CHEMBL3770342) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Antagonist activity at 5HT2B (unknown origin) expressed in CHOK1 cells assessed as inhibition of agonist-induced effect preincubated for 60 mins at 3... | ACS Med Chem Lett 9: 1019-1024 (2018) Article DOI: 10.1021/acsmedchemlett.8b00300 BindingDB Entry DOI: 10.7270/Q2DZ0C0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||