

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1CC(=O)OB(OC(=O)C1)c1ccc(Cn2c(C)nc3c(c(Cl)c(Cl)cc23)[N+]([O-])=O)cc1
InChI Key: InChIKey=FUJZJWQLQGXDEK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150052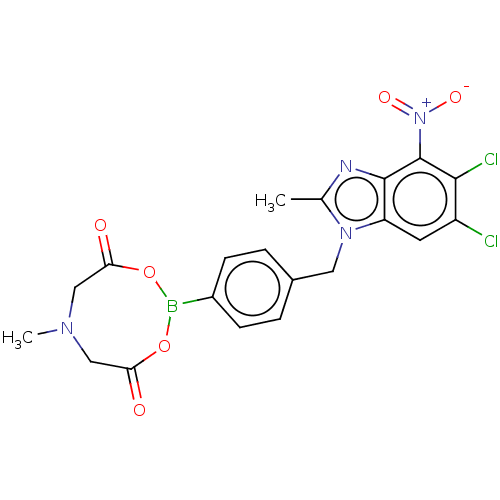 (CHEMBL3771105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human recombinant full length dCTPase expressed in Escherichia coli BL21(DE3)pLysS using dCTP as substrate after 1 hr by HTS-adapted Ma... | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate preincubated for 10 mins followed by NADPH addition by LC/MS/MS analysis | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using testostrone as substrate preincubated for 10 mins followed by NADPH addition by LC/MS/MS analysis | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dCTP pyrophosphatase 1 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of human dCTPase 1 | Bioorg Med Chem Lett 27: 3897-3904 (2017) Article DOI: 10.1016/j.bmcl.2017.06.038 BindingDB Entry DOI: 10.7270/Q23N25WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) using mephenytoin as substrate preincubated for 10 mins followed by NADPH addition by LC/MS/MS analysis | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) using bufuralol as substrate preincubated for 10 mins followed by NADPH addition by LC/MS/MS analysis | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) using phenacetin as substrate preincubated for 10 mins followed by NADPH addition by LC/MS/MS analysis | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50150052 (CHEMBL3771105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using diclofenac as substrate preincubated for 10 mins followed by NADPH addition by LC/MS/MS analysis | J Med Chem 59: 1140-8 (2016) BindingDB Entry DOI: 10.7270/Q2959KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||