

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50152974 (2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo[3,4-b]-[1,5]benzodiazepin-5(1H)-yl)carbonyl]benzyl}-2-[(4-methyl-1,4-diazepan-1-yl)carbonothioyl]pyrrolidine-1-carboxamide::(S)-2-(4-Methyl-[1,4]diazepane-1-carbothioyl)-pyrrolidine-1-carboxylic acid 2-methyl-4-(3-methyl-4,10-dihydro-3H-2,3,4,9-tetraaza-benzo[f]azulene-9-carbonyl)-benzylamide::CHEMBL360648
SMILES: CN1CCCN(CC1)C(=S)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1Cc2cnn(C)c2Nc2ccccc12
InChI Key: InChIKey=KSNHHKZYKYNBEI-NDEPHWFRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50152974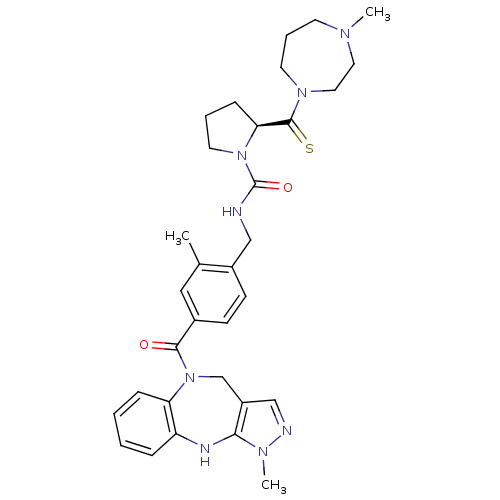 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of DY647 from SNAP-tagged OTR receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human oxytocin receptor expressed in CHO cells | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of DY647 from SNAP-tagged V1A receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1a receptor expressed in CHO cells | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of DY647 from SNAP-tagged V2 receptor (unknown origin) expressed in HEK293 cells by TR-FRET assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Agonist activity at human OTR receptor expressed in CHO cells co-expressing NFAT-luciferase incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Agonist activity at human OTR expressed in HEK cells assessed as increase in intracellular calcium flux incubated for 45 mins by Indo1-AM dye based f... | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Antagonist activity at human V1A receptor expressed in HEK cells assessed as inhibition of AVP-induced intracellular calcium flux incubated for 45 mi... | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Agonist activity at human V2 receptor expressed in CHO cells co-expressing CRE-luciferase incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Activity at human vasopressin V2 receptor expressed in CHO cells by NFAT-luciferase gene reporter assay | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Ferring Research Ltd Curated by ChEMBL | Assay Description Effective concentration for human Oxytocin receptor | Bioorg Med Chem Lett 14: 4585-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.107 BindingDB Entry DOI: 10.7270/Q2DF6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Ferring Research Ltd Curated by ChEMBL | Assay Description Effective concentration for human Vassopressin V2 receptor | Bioorg Med Chem Lett 14: 4585-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.107 BindingDB Entry DOI: 10.7270/Q2DF6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50152974 ((2S)-N-{2-Methyl-4-[(1-methyl-4,10-dihydropyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Activity at human oxytocin receptor expressed in CHO cells by NFAT-luciferase gene reporter assay | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||