

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50154850 CHEMBL3774588::US10273244, Example 22::US10584135, Example 22
SMILES: Cc1ncoc1-c1nnc(SCCCN2CC3CCN(C3C2)c2ccc(F)cc2)n1C
InChI Key: InChIKey=FZEUFNLUEJZENL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50154850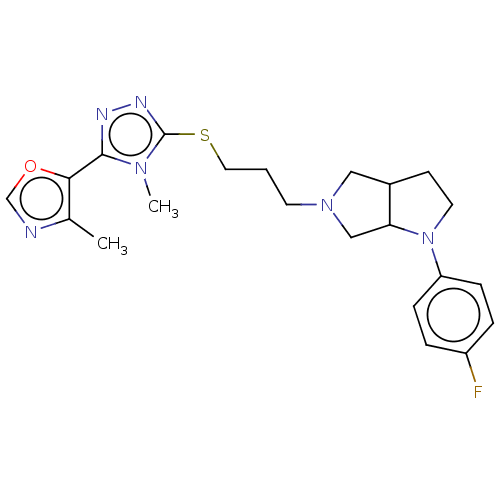 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counter metho... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counter metho... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description 3H]-Spiperone Binding Assay at hD3 and hD4 recombinant receptors CHO cells transiently transfected with human dopamine type 3 or 4 receptors (CHO-hD3... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2RN3B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description [3H]-Spiperone Binding Assay at hD3 and hD4 recombinant receptors CHO cells transiently transfected with human dopamine type 3 or 4 receptors (CHO-hD... | US Patent US10584135 (2020) BindingDB Entry DOI: 10.7270/Q29S1TFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counter metho... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were seeded into b... | US Patent US10584135 (2020) BindingDB Entry DOI: 10.7270/Q29S1TFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description Functional Calcium Assay at hD2 recombinant receptor. CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to G&#... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2RN3B5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes after 120 mins by liquid scintillation counter meth... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes after 120 mins by liquid scintillation counter meth... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes after 120 mins by liquid scintillation counter meth... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A2 expressed in baculosomes preincubated for 10 mins followed by cofactor addition measured every minute for 10 m... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2C19 expressed in baculosomes preincubated for 10 mins followed by cofactor addition measured every minute for 10 ... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of human ERG tail current expressed in HEK293 cells after 5 mins by patch clamp assay | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of human ERG tail current expressed in HEK293 cells after 5 mins by patch clamp assay | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2C9 expressed in baculosomes preincubated for 10 mins followed by cofactor addition measured every minute for 10 m... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 expressed in baculosomes preincubated for 10 mins followed by cofactor addition measured every minute for 10 m... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 expressed in baculosomes preincubated for 10 mins followed by cofactor addition measured every minute for 10 m... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50154850 (CHEMBL3774588 | US10273244, Example 22 | US1058413...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 expressed in baculosomes preincubated for 10 mins followed by cofactor addition measured every minute for 10 m... | Bioorg Med Chem 24: 1619-36 (2016) BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||