

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50159347 CHEMBL3787662::US9586965, Cpd 1
SMILES: CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cc1
InChI Key: InChIKey=HAJRCWPFNCVVCY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2/Receptor tyrosine-protein kinase erbB-3/Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50159347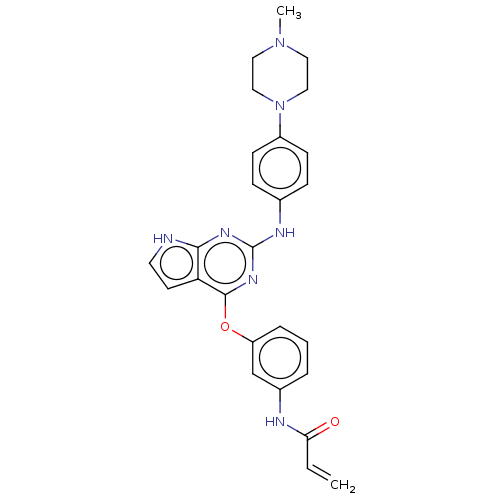 (CHEMBL3787662 | US9586965, Cpd 1) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis | J Med Chem 59: 2005-24 (2016) BindingDB Entry DOI: 10.7270/Q2KS6TDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2/Receptor tyrosine-protein kinase erbB-3/Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of EGFR L858R/T790M double mutant autophosphorylation in human NCI-H1975 cells after 2 hrs by sandwich ELISA | J Med Chem 59: 2005-24 (2016) BindingDB Entry DOI: 10.7270/Q2KS6TDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ACEA BIOSCIENCES INC. US Patent | Assay Description Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ... | US Patent US9586965 (2017) BindingDB Entry DOI: 10.7270/Q23R0VZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.579 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ACEA BIOSCIENCES INC. US Patent | Assay Description Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ... | US Patent US9586965 (2017) BindingDB Entry DOI: 10.7270/Q23R0VZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0366 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ACEA BIOSCIENCES INC. US Patent | Assay Description Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ... | US Patent US9586965 (2017) BindingDB Entry DOI: 10.7270/Q23R0VZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ACEA BIOSCIENCES INC. US Patent | Assay Description Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ... | US Patent US9586965 (2017) BindingDB Entry DOI: 10.7270/Q23R0VZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated wild type EGFR autophosphorylation expressed in human A549 cells by sandwich ELISA | J Med Chem 59: 2005-24 (2016) BindingDB Entry DOI: 10.7270/Q2KS6TDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50159347 (CHEMBL3787662 | US9586965, Cpd 1) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of dofetilide binding to human ERG | J Med Chem 59: 2005-24 (2016) BindingDB Entry DOI: 10.7270/Q2KS6TDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||