

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50160864 CHEMBL2373970::Fullerene Derivative
SMILES: [H][C@@]12C3=c4c5c6c7c8C(=C9c%10c1c1c%11c%12-c%13c%14c%15c%16c%17c%18c(c4c4c5c5c7c7c%19c8[C@]8%20[C@@H](N(CC(O)=O)C[C@]98[C@]([H])(c%10%12)[C@]8([H])c%13c9c%10c8c%20c%19c8c%10c%10c(c%159)c%16c9c%18c4c4c9c%10c8c7c54)C(O)=O)[C@@]([H])([C@@]31[H])[C@@]%17([H])[C@@]%11%14[H])[C@@]26[H]
InChI Key: InChIKey=JGQCYTLLVMXXQL-LLUJJWFISA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM50160864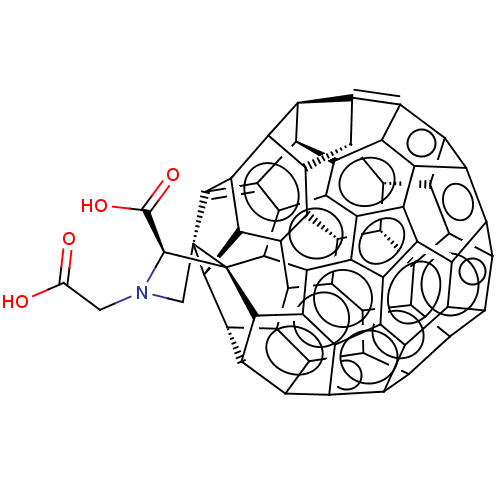 (CHEMBL2373970 | Fullerene Derivative) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoritsu University of Pharmacy Curated by ChEMBL | Assay Description Inhibitory concentration against Hepatitis C virus-RNA-dependent RNA polymerase | Bioorg Med Chem Lett 15: 1107-9 (2005) Article DOI: 10.1016/j.bmcl.2004.12.030 BindingDB Entry DOI: 10.7270/Q29C6WZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50160864 (CHEMBL2373970 | Fullerene Derivative) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoritsu University of Pharmacy Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-Reverse transcriptase | Bioorg Med Chem Lett 15: 1107-9 (2005) Article DOI: 10.1016/j.bmcl.2004.12.030 BindingDB Entry DOI: 10.7270/Q29C6WZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||