

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50165425 CHEMBL195963::[(S)-1-((R)-1-Phenyl-ethylaminooxalyl)-pentyl]-carbamic acid (S)-(1,2,3,4-tetrahydro-naphthalen-1-yl) ester
SMILES: CCCC[C@H](NC(=O)O[C@H]1CCCc2ccccc12)C(=O)C(=O)N[C@H](C)c1ccccc1
InChI Key: InChIKey=SDWKGFZATWAOLK-LEOXJPRUSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50165425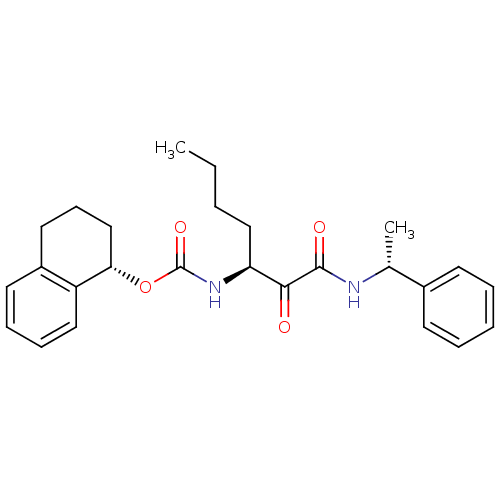 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H by fluorescence assay using 50 uM L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50165425 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin K | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50165425 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin L by fluorescence assay using 5 uM Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50165425 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin S by fluorescence assay using 10 uM Cbz-Val-Val-Arg-AMC | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50165425 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin B by fluorescence assay using 10 uM Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM50165425 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin V by fluorescence assay using 2 uM Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||