 Found 14 hits for monomerid = 50168472
Found 14 hits for monomerid = 50168472 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphoinositide 3-Kinase (PI3K), delta
(Homo sapiens (Human)) | BDBM50168472
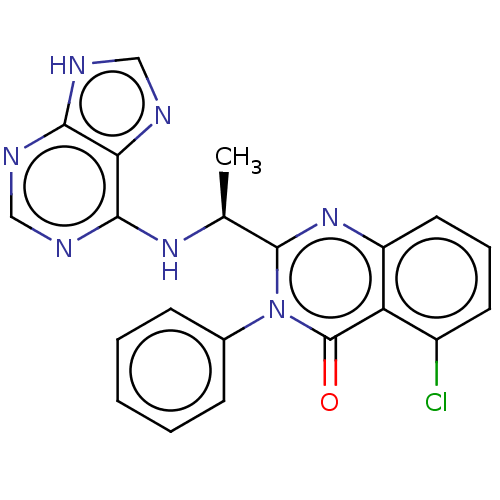
(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
TR-FRET monitored the formation of 3,4,5-inositol triphosphate molecule that competed with fluorescently labeled PIP3 for binding to the GRP-1 plecks... |
US Patent US9765060 (2017)
BindingDB Entry DOI: 10.7270/Q2DZ0BDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data