

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50170821 CHEMBL365026::N*6*-Cyclooctyl-N*2*-phenyl-9H-purine-2,6-diamine
SMILES: C1CCCC(CCC1)Nc1nc(Nc2ccccc2)nc2nc[nH]c12
InChI Key: InChIKey=MSEVQUIRSAPIPS-UHFFFAOYSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50170821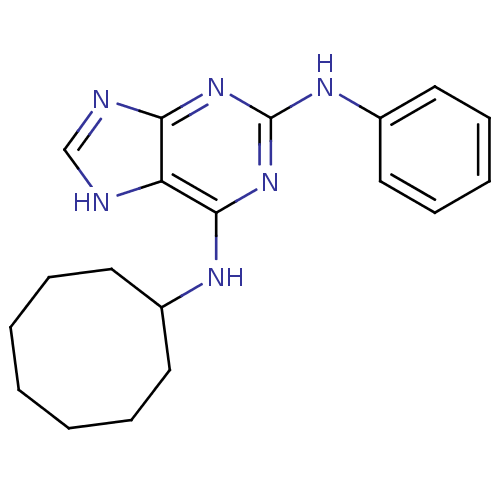 (CHEMBL365026 | N*6*-Cyclooctyl-N*2*-phenyl-9H-puri...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]- AB-MECA binding to human adenosine A3 receptor expressed in CHO cells | J Med Chem 48: 4910-8 (2005) Article DOI: 10.1021/jm050221l BindingDB Entry DOI: 10.7270/Q2N58KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50170821 (CHEMBL365026 | N*6*-Cyclooctyl-N*2*-phenyl-9H-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Percent inhibition of [3H]-DPCPX binding to human adenosine A1 receptor expressed in CHO cells at 10 uM | J Med Chem 48: 4910-8 (2005) Article DOI: 10.1021/jm050221l BindingDB Entry DOI: 10.7270/Q2N58KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50170821 (CHEMBL365026 | N*6*-Cyclooctyl-N*2*-phenyl-9H-puri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Percent inhibition of [3H]ZM241,385 binding to human adenosine A2a receptor expressed in CHO cells at 10 uM | J Med Chem 48: 4910-8 (2005) Article DOI: 10.1021/jm050221l BindingDB Entry DOI: 10.7270/Q2N58KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||