

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCN1c2ncccc2N(C)C(=O)c2cc(CCOc3ccc(cc3)C(O)=O)cnc12
InChI Key: InChIKey=KZFWFFCAGSLUEX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50171972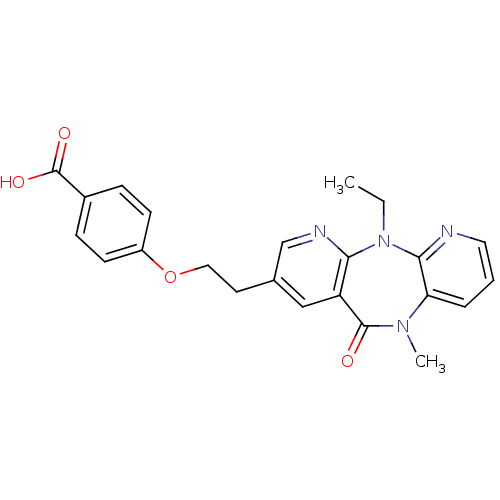 (4-[2-(5-Ethyl-10-methyl-11-oxo-10,11-dihydro-5H-4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of compound dissolved in DMSO was determined against HIV-1 (K103N/Y181C) mutant reverse transcriptase (1-2 nM) by using [3H]-dGTP... | J Med Chem 48: 5580-8 (2005) Article DOI: 10.1021/jm050255t BindingDB Entry DOI: 10.7270/Q29K49RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50171972 (4-[2-(5-Ethyl-10-methyl-11-oxo-10,11-dihydro-5H-4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Effective concentration towards HIV-1 wild type reverse transcriptase activity by 50% measured in cellular assay | J Med Chem 48: 5580-8 (2005) Article DOI: 10.1021/jm050255t BindingDB Entry DOI: 10.7270/Q29K49RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50171972 (4-[2-(5-Ethyl-10-methyl-11-oxo-10,11-dihydro-5H-4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 162 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Effective concentration of the compound towards HIV-1 K103N/Y181C mutant reverse transcriptase activity by 50% measured in cellular assay | J Med Chem 48: 5580-8 (2005) Article DOI: 10.1021/jm050255t BindingDB Entry DOI: 10.7270/Q29K49RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50171972 (4-[2-(5-Ethyl-10-methyl-11-oxo-10,11-dihydro-5H-4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of compound dissolved in DMSO was determined against HIV-1 wild type reverse transcriptase (1-2 nM) by using [3H]-dGTP (71 nM) a... | J Med Chem 48: 5580-8 (2005) Article DOI: 10.1021/jm050255t BindingDB Entry DOI: 10.7270/Q29K49RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||