

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50172138 CHEMBL3808443::US10040788, Example 1(a)::US10294221, Example 1(a)
SMILES: CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2nccnn2)s1)c1ccccc1
InChI Key: InChIKey=YPVDEDXOCCSSBO-KGLIPLIRSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50172138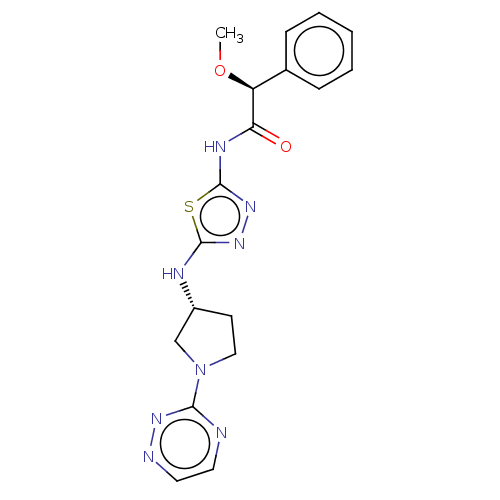 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of 6His-tagged GLS1 (63-669 residues) (unknown origin) expressed in Escherichia coli using glutamine as substrate after 15 mins by Glutama... | ACS Med Chem Lett 7: 207-8 (2016) BindingDB Entry DOI: 10.7270/Q2GX4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50172138 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of GLS1 in human PC3 cells assessed as cellular glutamate depletion levels after 6 hrs by Glutamate Oxidase/ AmplexRed coupled assay | ACS Med Chem Lett 7: 207-8 (2016) BindingDB Entry DOI: 10.7270/Q2GX4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50172138 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 62.8 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50172138 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50172138 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assay | J Med Chem 62: 46-59 (2019) Article DOI: 10.1021/acs.jmedchem.8b00327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50172138 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by Ionworks electrophysiology assay | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50172138 (CHEMBL3808443 | US10040788, Example 1(a) | US10294...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 62.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||