

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50173419 (3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-trifluoromethyl-phenyl)-pyridin-2-yl]-vinyl}-decahydro-naphtho[2,3-c]furan-1-one::(3R,3aS,4S,4aR,8aS,9aR)-3-methyl-4-(2-(5-(3-(trifluoromethyl)phenyl)pyridin-2-yl)vinyl)-decahydronaphtho[2,3-c]furan-1(3H)-one::(3R,3aS,4S,4aR,8aS,9aR,E)-3-methyl-4-(2-(5-(3-(trifluoromethyl)phenyl)pyridin-2-yl)vinyl)-decahydronaphtho[2,3-c]furan-1(3H)-one::CHEMBL371069::SCH-205831
SMILES: C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12
InChI Key: InChIKey=PQLBJVPZXNPVOS-HLBWOJLBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419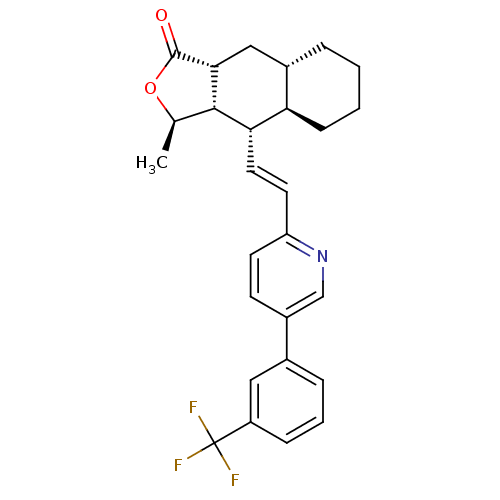 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory constant aganist Protease-activated receptor 1 | J Med Chem 48: 5884-7 (2005) Article DOI: 10.1021/jm0502236 BindingDB Entry DOI: 10.7270/Q2V69J5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration aganist human protease activated receptor 1 using [3H]-haTRAP as radioligand | J Med Chem 48: 5884-7 (2005) Article DOI: 10.1021/jm0502236 BindingDB Entry DOI: 10.7270/Q2V69J5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from human PAR1 receptor in platelet membrane | Bioorg Med Chem Lett 17: 4509-13 (2007) Article DOI: 10.1016/j.bmcl.2007.06.002 BindingDB Entry DOI: 10.7270/Q2RN37J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to PAR1 in human platelet membrane | Bioorg Med Chem Lett 16: 4969-72 (2006) Article DOI: 10.1016/j.bmcl.2006.06.042 BindingDB Entry DOI: 10.7270/Q2CV4HC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR1 in human platelet membrane | J Med Chem 50: 129-38 (2007) Article DOI: 10.1021/jm061043e BindingDB Entry DOI: 10.7270/Q22V2FS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration aganist human protease activated receptor 1 using [3H]-haTRAP as radioligand | J Med Chem 48: 5884-7 (2005) Article DOI: 10.1021/jm0502236 BindingDB Entry DOI: 10.7270/Q2V69J5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50173419 ((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration aganist human protease activated receptor 1 using [3H]-haTRAP as radioligand | J Med Chem 48: 5884-7 (2005) Article DOI: 10.1021/jm0502236 BindingDB Entry DOI: 10.7270/Q2V69J5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||