

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(cc1)N1N=C(\C(=C\c2ccc(o2)-c2ccccc2C(O)=O)C1=O)c1ccccc1
InChI Key: InChIKey=SKEVDQNJWPPYIJ-ULJHMMPZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50176860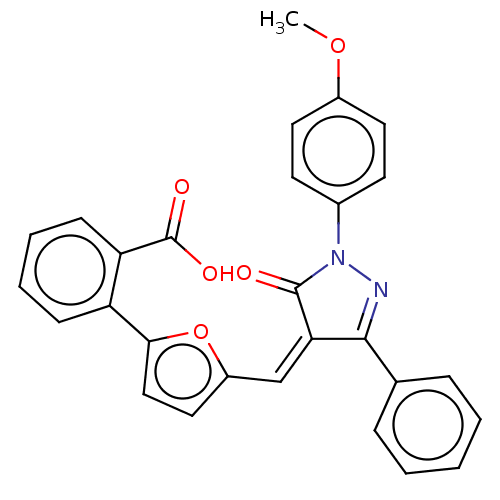 (CHEMBL3809059) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus recombinant 3CL-PRO expressed in Escherichia coli JM109 cells using Dabcyl-KTSAVLQSGFRKME-Edans as fluorogenic substra... | Bioorg Med Chem 24: 3035-3042 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5JK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||