

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50177408 4-(hydroxymethyl)phenol::4-Hydroxybenzyl alcohol::4HBA::CHEMBL202132::parahydroxybenzyl alcohol
SMILES: OCc1ccc(O)cc1
InChI Key: InChIKey=BVJSUAQZOZWCKN-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosinase (Mus musculus (Mouse)) | BDBM50177408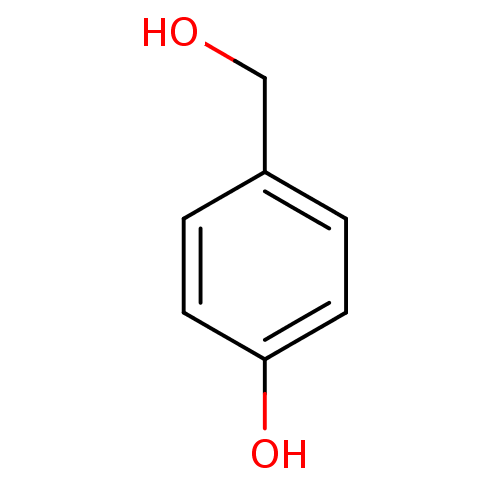 (4-(hydroxymethyl)phenol | 4-Hydroxybenzyl alcohol ...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | 6.8 | 25 |
National Tsing Hua University | Assay Description The enzyme activity was monitored by dopachrome formation at 475nm for an appropriate period. | J Enzyme Inhib Med Chem 23: 526-34 (2008) Article DOI: 10.1080/14756360701654894 BindingDB Entry DOI: 10.7270/Q29885MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50177408 (4-(hydroxymethyl)phenol | 4-Hydroxybenzyl alcohol ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | 6.8 | 25 |
National Tsing Hua University | Assay Description The enzyme activity was monitored by dopachrome formation at 475nm for an appropriate period. | J Enzyme Inhib Med Chem 23: 526-34 (2008) Article DOI: 10.1080/14756360701654894 BindingDB Entry DOI: 10.7270/Q29885MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50177408 (4-(hydroxymethyl)phenol | 4-Hydroxybenzyl alcohol ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Plant Development Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA after 10 mins by ELISA reader | J Nat Prod 74: 1009-14 (2011) Article DOI: 10.1021/np100900k BindingDB Entry DOI: 10.7270/Q2TQ61WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-amino-N-butyrate transaminase (Homo sapiens (Human)) | BDBM50177408 (4-(hydroxymethyl)phenol | 4-Hydroxybenzyl alcohol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity against GABAT | Bioorg Med Chem Lett 16: 592-5 (2005) Article DOI: 10.1016/j.bmcl.2005.10.040 BindingDB Entry DOI: 10.7270/Q29G5MCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Succinate semialdehyde dehydrogenase (Homo sapiens (Human)) | BDBM50177408 (4-(hydroxymethyl)phenol | 4-Hydroxybenzyl alcohol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity against SSADH | Bioorg Med Chem Lett 16: 592-5 (2005) Article DOI: 10.1016/j.bmcl.2005.10.040 BindingDB Entry DOI: 10.7270/Q29G5MCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||