

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50181364 2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-piperidine)-1-ylpropyl]benzamide::CHEMBL201805
SMILES: O=C(NCCCN1CCC2(CCc3ccccc23)CC1)c1ccccc1OCc1ccccc1
InChI Key: InChIKey=BZYCJZFLTTYKQH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181364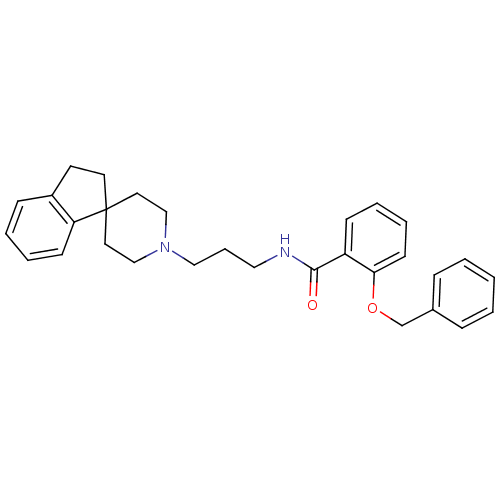 (2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50181364 (2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from cloned human delta opioid receptor expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50181364 (2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from cloned human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50181364 (2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphin from cloned human mu opioid receptor expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181364 (2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50181364 (2-Benzyloxy-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Agonist activity on nociceptin-induced maximal [35S]GTP-gamma-S binding to ORL1 expressed in CHO cells | J Med Chem 49: 847-9 (2006) Article DOI: 10.1021/jm0509851 BindingDB Entry DOI: 10.7270/Q2DJ5F78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||