

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1CCN(CCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1
InChI Key: InChIKey=JNAZGEGXKHNDBG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakpoint cluster region protein/Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50181672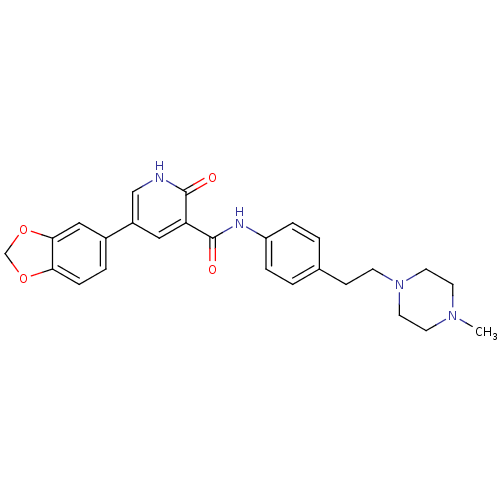 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Antiproliferative activity against human BCR-ABL positive chronic myeloid leukemia K562 cell line | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor/Nucleophosmin (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Antiproliferative activity against human NPM-ALK positive anaplastic large cell lymphoma karpas299 cell line | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against ALK | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor/Nucleophosmin (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Antiproliferative activity against murine BaF3 cell line expressing NPM-ALK | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against IGF1R | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against Flt3 | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against Src | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against Abl | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50181672 (5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation Curated by ChEMBL | Assay Description Inhibitory activity against IRK | J Med Chem 49: 1006-15 (2006) Article DOI: 10.1021/jm050824x BindingDB Entry DOI: 10.7270/Q2H994T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||