

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50182250 CHEMBL3819521
SMILES: CN1CCC(CC1)S(=O)(=O)c1c(Cl)ccc(NC(=O)Nc2cccc(Cl)c2Cl)c1O
InChI Key: InChIKey=ITIUSBGOKOUQFX-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50182250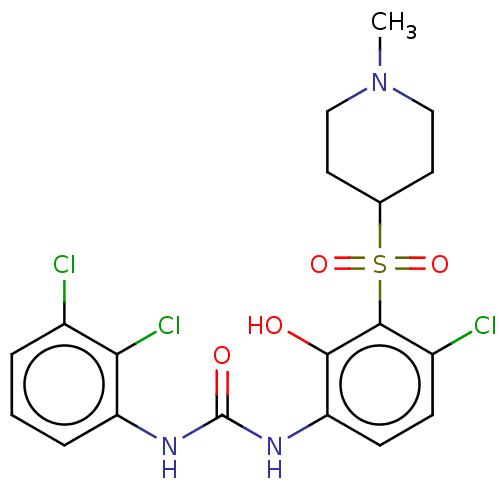 (CHEMBL3819521) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50182250 (CHEMBL3819521) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50182250 (CHEMBL3819521) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human recombinant Gal4-VP16 fused-CXCR2 assessed as inhibition of CXCL1-mediated lactamase reporter gene expression after over... | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50182250 (CHEMBL3819521) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 assessed as inhibition of CXCL1-induced neutrophil chemotaxis after 45 mins by calcein-AM dye based pl... | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50182250 (CHEMBL3819521) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50182250 (CHEMBL3819521) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50182250 (CHEMBL3819521) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50182250 (CHEMBL3819521) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College and Chinese Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 397-402 (2016) BindingDB Entry DOI: 10.7270/Q2KW5HZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||