

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50185042 CHEMBL206081::N-[1-(1,3-benzodioxol-5-ylmethyl)piperidin-4-yl]-7-methoxy-4-oxo-4H-chromene-2-carboxamide
SMILES: COc1ccc2c(c1)oc(cc2=O)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1
InChI Key: InChIKey=BLVUILRZDGFJPZ-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanin-concentrating hormone receptor (Homo sapiens (Human)) | BDBM50185042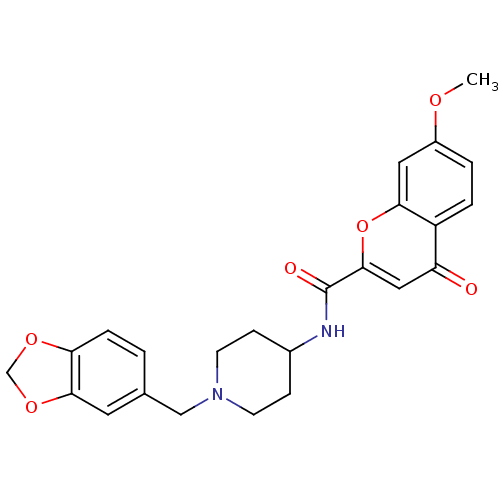 (CHEMBL206081 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from MCHr1 expressed in IMR32 (I3.4.2) cells | J Med Chem 49: 2339-52 (2006) Article DOI: 10.1021/jm0512286 BindingDB Entry DOI: 10.7270/Q24F1Q9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50185042 (CHEMBL206081 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from hERG | J Med Chem 49: 6569-84 (2006) Article DOI: 10.1021/jm060683e BindingDB Entry DOI: 10.7270/Q2NG4Q7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor (Homo sapiens (Human)) | BDBM50185042 (CHEMBL206081 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at MCHr1 assessed as inhibition of MCH-mediated calcium ion release in intact IMR32 cells by FLIPR assay | J Med Chem 49: 6569-84 (2006) Article DOI: 10.1021/jm060683e BindingDB Entry DOI: 10.7270/Q2NG4Q7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor (Homo sapiens (Human)) | BDBM50185042 (CHEMBL206081 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells | J Med Chem 49: 6569-84 (2006) Article DOI: 10.1021/jm060683e BindingDB Entry DOI: 10.7270/Q2NG4Q7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||